Abstract
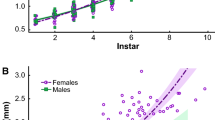
In organisms with determinate growth, sexual size dimorphism (SSD) occurs before maturity during the developmental process of growing apart, an ontogenetic perspective on the evolution of SSD. If the direction of SSD (female-larger SSD) is known, patterns of growth can be tested with one-tailed statistical distributions. In indeterminate growing organisms as well, does SSD occur before maturity? If it occurs, whether is females’ larger mean body size caused by a difference in age at maturity, age-specific size, divergent growth prior to maturity, or selection on post-maturational growth? How important is biphasic, sexual shape dimorphism (BSSD) for determinants of SSD? Biphasic characteristics are those that differ between adult aquatic- and terrestrial-phase morphs, and shape is size of a characteristic relative to body size. To address those questions, I determined age and body size based on a careful description of a growth trajectory for each sex in Salamandrella keyserlingii, using 555 independent data points from skeletochronological studies. Females reached maturity at 3–4 years of age, a year later than males that reached maturity at 2–3 years of age (mean body size: males = 57.63 mm, females = 61.70 mm; delayed sexual maturity resulted in SSD). However, SSD was highly detected before maturity (SSD index = 0.097), and females after maturity continued to grow and resulted in larger asymptotic size than males. Traits of BSSD were greater in males than in females. These results suggest that when determining SSD the difference in mean adult-body size results from the difference in age-specific size and the female-larger SSD develops to resolve intersexual ontogenetic conflict by allowing small-sized males to swell their whole body during the aquatic phase as much as large-sized females.




Similar content being viewed by others
References
Andersson, M. (1994). Sexual selection. Princeton, NJ: Princeton University Press.
Arntzen, J. W. (2000). A growth curve for the newt Triturus cristatus. Journal of Herpetology, 34, 227–232.
Badyaev, A. V. (2002). Growing apart: An ontogenetic perspective on the evolution of sexual size dimorphism. Trends in Ecology & Evolution, 17, 369–378.
Barot, S., Heino, M., O’Brien, L., & Dieckmann, U. (2004). Estimating reaction norms for age and size at maturation when age at first reproduction is unknown. Evolutionary Ecology Research, 6, 659–678.
Blackwell, E. A., Angus, R. A., Cline, G. R., & Marion, K. R. (2003). Natural growth rates of Ambystoma maculatum in Alabama. Journal of Herpetology, 37, 608–612.
Borkin, L. (1999). Salamandrella keyserlingii Dybowski, 1870. Sibirischer Winkelzahnmolch. In K. Grossenbacher & B. Thiesmeier (Eds.), Handbuch der Reptilien und Amphibien Europas, Vol. 4/1, Urodela 1 (pp. 21–55). Wiesbaden, Hessen, Deutschland: Aula-Verlag.
Bruce, R. C. (1993). Sexual size dimorphism in desmognathine salamanders. Copeia, 1993, 313–318.
Bruce, R. C. (2000). Sexual size dimorphism in the Plethodontidae. In R. C. Bruce, R. G. Jaeger, & L. D. Houck (Eds.), The biology of plethodontid salamanders (pp. 243–260). New York: Kluwer Academic/Plenum Publishers.
Caetano, M. H., & Castanet, J. (1993). Variability and microevolutionary patterns in Triturus marmoratus from Portugal: Age, size, longevity and individual growth. Amphibia-Reptilia, 14, 117–129.
Charnov, E. L. (1993). Life history invariants. Oxford: Oxford University Press.
Cox, R. M., & John-Alder, H. B. (2007). Growing apart together: The development of contrasting sexual size dimorphisms in systematic Sceloporus lizards. Herpetologica, 63, 245–257.
Czarnoleski, M., & Kozlowski, J. (1998). Do Bertalanffy’s growth curves result from optimal resource allocation? Ecology Letters, 1, 5–7.
Day, T., & Taylor, P. D. (1997). Von Bertalanffy’s growth equation should not be used to model age and size at maturity. American Naturalist, 149, 381–393.
Duellman, W. E., & Trueb, L. (1986). Biology of amphibians. New York: McGraw-Hill.
Dunham, A. E. (1978). Food availability as a proximate factor influencing individual growth rates in the iguanid lizard Sceloporus merriami. Ecology, 59, 770–778.
Eden, C. J., Whiteman, H. H., Duobinis-Gray, L., & Wissinger, S. A. (2007). Accuracy assessment of skeletochronology in the Arizona tiger salamander (Ambystoma tigrinum nebulosum). Copeia, 2007, 471–477.
Francillon-Vieillot, H., Arntzen, J. W., & Géraudie, J. (1990). Age, growth and longevity of sympatric Triturus cristatus, T. marmoratus and their hybrids (Amphibia, Urodela): A skeletochronological comparison. Journal of Herpetology, 24, 13–22.
Griffiths, A. D., & Brook, B. W. (2005). Body size and growth in tropical small mammals: Examining variation using non-linear mixed effects models. Journal of Zoology (London), 267, 211–220.
Halliday, T., & Tejedo, M. (1995). Intrasexual selection and alternative mating behaviour. In H. Heatwole & B. K. Sullivan (Eds.), Amphibian biology, Vol. 2, Social behaviour (pp. 419–468). Chipping Norton, New South Wales, Australia: Surrey Beatty and Sons.
Halliday, T. R., & Verrell, P. A. (1988). Body size and age in amphibians and reptiles. Journal of Herpetology, 22, 253–265.
Hasumi, M. (1994). Reproductive behavior of the salamander Hynobius nigrescens: Monopoly of egg sacs during scramble competition. Journal of Herpetology, 28, 264–267.
Hasumi, M. (1996). Times required for ovulation, egg sac formation, and ventral gland secretion in the salamander Hynobius nigrescens. Herpetologica, 52, 605–611.
Hasumi, M. (2001a). Sexual behavior in female-biased operational sex ratios in the salamander Hynobius nigrescens. Herpetologica, 57, 396–406.
Hasumi, M. (2001b). Secondary sexual characteristics of the salamander Salamandrella keyserlingii: Throat coloration. Herpetological Review, 32, 223–225.
Hasumi, M., Hongorzul, T., & Terbish, K. (2009). Burrow use by Salamandrella keyserlingii (Caudata: Hynobiidae). Copeia, 2009, 46–49.
Hasumi, M., & Iwasawa, H. (1990). Seasonal changes in body shape and mass in the salamander, Hynobius nigrescens. Journal of Herpetology, 24, 113–118.
Hasumi, M., & Kanda, F. (1998). Breeding habitats of the Siberian salamander (Salamandrella keyserlingii) within a fen in Kushiro Marsh, Japan. Herpetological Review, 29, 150–153.
Hasumi, M., & Kanda, F. (2007). Phenological activity estimated by movement patterns of the Siberian salamander near a fen. Herpetologica, 63, 163–175.
Hasumi, M., & Watanabe, Y. G. (2007). An efficient method for skeletochronology. Herpetological Review, 38, 404–406.
Heino, M., & Kaitala, V. (1999). Evolution of resource allocation between growth and reproduction in animals with indeterminate growth. Journal of Evolutionary Biology, 12, 423–429.
Hemelaar, A. (1988). Age, growth and other population characteristics of Bufo bufo from different latitudes and altitudes. Journal of Herpetology, 22, 369–388.
John-Alder, H. B., Cox, R. M., & Taylor, E. N. (2007). Proximate developmental mediators of sexual dimorphism in size: Case studies from squamate reptiles. Integrative and Comparative Biology, 47, 258–271.
Kirkpatrick, M. (1984). Demographic models based on size, not age, for organisms with indeterminate growth. Ecology, 65, 1874–1884.
Kozlowski, J., & Uchmañski, J. (1987). Optimal individual growth and reproduction in perennial species with indeterminate growth. Evolutionary Ecology, 1, 214–230.
Kusano, T., Ueda, T., & Nakagawa, H. (2006). Body size and age structure of breeding populations of the salamander, Hynobius tokyoensis (Caudata: Hynobiidae). Current Herpetology, 25, 71–78.
Kuzmin, S. L. (1994). The geographical range of Salamandrella keyserlingii: Ecological and historical implications. Abhandlungen und Berichte für Naturkunde, 17, 177–183.
Kyriakopoulou-Sklavounou, P., Stylianou, P., & Tsiora, A. (2008). A skeletochronological study of age, growth and longevity in a population of the frog Rana ridibunda from southern Europe. Zoology, 111, 30–36.
Leclair, M. H., Levasseur, M., & Leclair, R., Jr. (2006). Life-history traits of Plethodon cinereus in the northern parts of its range: Variations in population structure, age and growth. Herpetologica, 62, 265–282.
Lester, N. P., Shuter, B. J., & Abrams, P. A. (2004). Interpreting the von Bertalanffy model of somatic growth in fishes: The cost of reproduction. Proceedings of the Royal Society of London B, Biological Sciences, 271, 1625–1631.
Lovich, J. E., & Gibbons, J. W. (1992). A review of techniques for quantifying sexual size dimorphism. Growth, Development, and Aging, 56, 269–281.
Maerz, J. C., Myers, E. M., & Adams, D. C. (2006). Trophic polymorphism in a terrestrial salamander. Evolutionary Ecology Research, 8, 23–35.
Maiorana, V. C. (1976). Size and environmental predictability for salamanders. Evolution, 30, 599–613.
Malmgren, J. C., & Thollesson, M. (1999). Sexual size and shape dimorphism in two species of newts, Triturus cristatus and T. vulgaris (Caudata: Salamandridae). Journal of Zoology (London), 249, 127–136.
Marangoni, F., Schaefer, E., Cajade, R., & Tejedo, M. (2009). Growth-mark formation and chronology of two neotropical anuran species. Journal of Herpetology, 43, 546–550.
Marvin, G. A. (2001). Age, growth, and long-term site fidelity in the terrestrial plethodontid salamander Plethodon kentucki. Copeia, 2001, 108–117.
Marvin, G. A. (2009). Sexual and seasonal dimorphism in the Cumberland Plateau woodland salamander, Plethodon kentucki (Caudata: Plethodontidae). Copeia, 2009, 227–232.
McKenzie, J., Page, B., Goldsworthy, S. D., & Hindell, M. A. (2007). Growth strategies of New Zealand fur seals in southern Australia. Journal of Zoology (London), 272, 377–389.
Miaud, C., Andreone, F., Ribéron, A., De Michelis, S., Clima, V., Castanet, J., et al. (2001). Variations in age, size at maturity and gestation duration among two neighbouring populations of the alpine salamander (Salamandra lanzai). Journal of Zoology (London), 254, 251–260.
Miaud, C., Guyétant, R., & Elmberg, J. (1999). Variation in life-history traits in the common frog Rana temporaria (Amphibia: Anura): A literature review and new data from the French Alps. Journal of Zoology (London), 249, 61–73.
Miaud, C., Guyetant, R., & Faber, H. (2000). Age, size, and growth of the Alpine newt, Triturus alpestris (Urodela: Salamandridae), at high altitude and a review of life-history trait variation throughout its range. Herpetologica, 56, 135–144.
Monnet, M. J., & Cherry, M. I. (2002). Sexual size dimorphism in anurans. Proceedings of the Royal Society of London B, Biological Sciences, 269, 2301–2307.
Olgun, K., Miaud, C., & Gautier, P. (2001). Age, growth, and survivorship in the viviparous salamander Mertensiella luschani from southwestern Turkey. Canadian Journal of Zoology, 79, 1559–1567.
Olsson, M., & Shine, R. (1996). Does reproductive success increase with age or with size in species with indeterminate growth? A case study using sand lizards (Lacerta agilis). Oecologia, 105, 175–178.
Pough, F. H., Andrews, R. M., Cadle, J. E., Crump, M. L., Savitzky, A. H., & Wells, K. D. (2001). Herpetology (2nd ed.). Upper Saddle River, NJ: Prentice-Hall.
Salthe, S. N. (1969). Reproductive modes and the numbers and sizes of ova in the urodeles. American Midland Naturalist, 81, 467–490.
Salvidio, S., & Bruce, R. C. (2006). Sexual dimorphism in two species of European plethodontid salamanders, genus Speleomantes. Herpetological Journal, 16, 9–14.
Scott, D. A. (1991). Asia and the Middle East. In M. Finlayson & M. Moser (Eds.), Wetlands (pp. 149–178). Oxford: Facts On File.
Tarling, G. A., & Cuzin-Roudy, J. (2003). Synchronization in the molting and spawning activity of northern krill (Meganyctiphanes norvegica) and its effect on recruitment. Limnology and Oceanography, 48, 2020–2033.
Tsiora, A., & Kyriakopoulou-Sklavounou, P. (2002). A skeletochronological study of age and growth in relation to adult size in the water frog Rana epeirotica. Zoology, 105, 55–60.
Verrell, P. A., & Davis, K. (2003). Do non-breeding, adult long-toed salamanders respond to conspecifics as friends or as foes? Herpetologica, 59, 1–7.
von Bertalanffy, L. (1938). A quantitative theory of organic growth (inquiries on growth laws. II). Human Biology, 10, 181–213.
Williams, R. N., & DeWoody, J. A. (2009). Reproductive success and sexual selection in wild eastern tiger salamanders (Ambystoma t. tigrinum). Evolutionary Biology, 36, 201–213.
Wise, S. E., & Buchanan, B. W. (1992). An efficient method for measuring salamanders. Herpetological Review, 23, 56–57.
Zeleznik, F. J. (1968). Quasi-Newton methods for nonlinear equations. Journal of the Association for Computing Machinery, 15, 265–271.
Acknowledgments
Cordial thanks are due to F. Kanda and all staff members of Onnenai Visitor Center, Kushiro Shitsugen National Park, for their partial support during my stay in Kushiro, and T. Kusano for discussing on independence of data. I am indebted to T. Halliday, C. Miaud, and D. Sever for critically reviewing the manuscript. I express my gratitude for the constructive comments of an onymous reviewer, J. Malmgren, and an anonymous reviewer. Handling of S. keyserlingii is regulated by the Government of Kushiro-shi, and this study was conducted under the permission authorized by this government. This study was financially supported in part by Grants-in-Aid for Scientific Research from the Japanese Foundation for the Management of Riparian Environments, the Maeda Ippo-en Foundation (Japan), and the Akiyama Memorial Foundation (Japan) for the Promotion of Life Sciences.
Author information
Authors and Affiliations
Corresponding author
Appendix 1
Appendix 1
See Table 4.
Rights and permissions
About this article
Cite this article
Hasumi, M. Age, Body Size, and Sexual Dimorphism in Size and Shape in Salamandrella keyserlingii (Caudata: Hynobiidae). Evol Biol 37, 38–48 (2010). https://doi.org/10.1007/s11692-010-9080-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-010-9080-9