Abstract
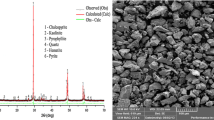
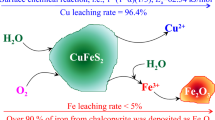
The recovery of copper from chalcopyrite by leaching is complex not only due to the slow dissolution kinetics of this mineral in most aqueous media but also due to the production of solutions that are heavily contaminated with iron. On the contrary, the leaching of sulfidized chalcopyrite is very attractive because of a faster and more selective dissolution of copper compared to the leaching of the untreated chalcopyrite. In this work, the results of leaching in H2SO4-NaCl-O2 solutions of sulfidized chalcopyrite concentrate are discussed. Experiments were carried out with chalcopyrite concentrates previously reacted with elemental sulfur at 375 °C for 60 minutes. The results showed that the concentration of chloride ions below 0.5 M, temperature, and leaching time are important variables for the extraction of Cu. On the other hand, Fe extraction was little affected by the same variables, remaining below 6 pct for all the experimental conditions tested. Microscopic observations of the leached particles showed that the elemental sulfur produced by the reaction does not form a coherent layer surrounding the particle, but rather concentrates in certain locations as large clusters. The leaching kinetics can be accurately described by a nonreactive core-shrinking rim topochemical expression for spherical particles 1 − (1 − 0.45X)1/3=kt. The activation energy found was 76 kJ/mol for the range 85 °C to 100 °C.
Similar content being viewed by others
References
R. Padilla, M. Torres, and M.C. Ruiz: EPD Congr., 2001, P. Taylor, ed., TMS, Warrendale, PA, 2001, pp. 457–69.
R. Padilla, E. Olivares, M.C. Ruiz, and H.Y. Sohn: Metall. Mater. Trans. B, 2003, vol. 34B, pp. 61–68.
I.H. Warren, A. Vizsolyi, and F.A. Forward: CIM Bull., 1968, vol. 61, pp. 637–40.
K.N. Subramanian and H. Kanduth: CIM Bull., 1973, vol. 66, pp. 88–91.
K.J. Cathro: Proc. Aus. Inst. Min. Metall., 1974, vol. 252, pp. 1–11.
G.P. Demopoulos and P.A. Distin: Hydrometallurgy, 1983, vol. 10, pp. 111–22.
C.Y. Cheng and F. Lawson: Hydrometallurgy, 1991, vol. 27, pp. 249–68.
C.Y. Cheng and F. Lawson: Hydrometallurgy, 1991, vol. 27, pp. 269–84.
T. Deng, Y. Lu, Z. Wen, and D. Liu: Hydrometallurgy, 2001, vol. 62, pp. 23–30.
Z.Y. Lu, M.I. Jeffrey, and F. Lawson: Hydrometallurgy, 2000, vol. 56, pp. 189–202.
L.W. Beckstead, P.B. Munoz, J.L. Sepulveda, J.A. Herbst, J.D. Miller, F.A. Olson, and M.E. Wadsworth: in Extractive Metallurgy of Copper, J.C. Yannopoulus and J.C. Agarwal, eds., AIME, New York, NY, 1976, pp. 611–32.
F. Lawson, C.Y. Cheng, and L.S.Y. Lee: Min. Processing Extr. Metall. Rev., 1992, vol. 8, pp. 183–203.
M.E. Wadsworth: in Rate Processes of Extractive Metallurgy, H.Y. Sohn and M.E. Wadsworth, eds., Plenum Press, New York, NY, 1979, pp. 133–86.
J. Szekely, J.W. Evans, and H.Y. Sohn: Gas-Solid Reactions, Academic Press, New York, NY, 1976, pp. 75–84.
E. Narita, F. Lawson, and K.N. Han: Hydrometallurgy, 1983, vol. 10, pp. 27–37.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Padilla, R., Zambrano, P. & Ruiz, M.C. Leaching of sulfidized chalcopyrite with H2SO4-NaCl-O2 . Metall Mater Trans B 34, 153–159 (2003). https://doi.org/10.1007/s11663-003-0002-4
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11663-003-0002-4