Abstract
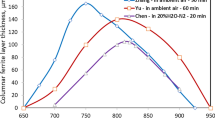
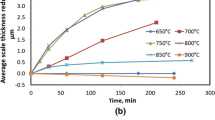
The decarburization behavior of a spring steel 60Si2MnA at 700 °C to 900 °C was examined. It was observed that after holding for 20 minutes in 20 pct H2O-N2, thick ferrite layers developed within 750 °C to 877 °C with a maximum thickness of about 100 μm observed at 805 °C to 825 °C, while the ferrite layers were much thinner at 900 °C and 700 °C. Carbon permeability analysis and theoretical calculation were conducted to assess the possibility of forming a ferrite layer. In the permeability analysis, several factors were considered: (1) carbon concentration at the steel surface, which was very likely determined by reaction equilibrium between FeO and dissolved carbon in steel, (2) carbon solubility in ferrite which had a maximum value at about 715 °C, and (3) carbon diffusivity in the ferrite phase. In the ferrite layer thickness calculation, the contribution from carbon diffusion in the austenite phase was also taken into account. While the carbon permeability analysis and ferrite layer thickness calculation showed good successes in predicting the pattern of ferrite layer thickness change with temperature, under the assumption of FeO-ferrite equilibrium the calculated ferrite layer thicknesses at 780 °C to 840 °C did not match the observed values well. Factors contributing to the discrepancy were discussed.











Similar content being viewed by others
References
GB/T 1222 – 2016. Spring Steels, National Standards of the People’s Republic of China (2016)
Designation: A29/A29M – 05. Standard specification for steel bars, carbon and alloy, hot-wrought, general requirement for. ASTM International (2006)
BS EN 10089:2002. Hot rolled steels for quenched and tempered springs – Technical delivery conditions, BSi British Standards (2002)
4. A. S. Kenneford, Journal of Iron and Steel Institute, 1950, vol. 164, pp. 265-77.
5 M. J. Geldersleeve, Materials Science and Technology, 1991, vol. 7, pp. 307 – 10.
6. M. Nomura, H. Morimoto and M. Toyama, ISIJ International, 2000, vol. 40, pp. 619 - 23.
D. Li, D. Anghelina, D. Burzic, J. Zamberger, R. Kienreich, H. Schifferi, W. Krieger and E. Kozeschnik, Steel Research International 2009, vol. 80, pp. 298 – 303
D. Li, D. Anghelina, D. Burzic, W. Krieger, E. Kozeschnik, Steel Research International, 2009, vol. 80, pp. 304-10.
8. C. Zhang, Y. Liu, L. Zhou, C. Jiang and J. Xiao, International Journal of Minerals, Metallurgy and Materials, 2012, vol. 19, pp. 116-21.
9. C. Zhang, L. Zhou, and Y. Liu, International Journal of Minerals, Metallurgy and Materials, 2013, vol. 20, pp. 720-24.
10. S. Choi and Y. Lee, ISIJ International, 2014, vol. 54, 1682 – 89.
11. S. Choi and S. Zwaag, ISIJ International, 2012, vol. 52, pp. 549 – 58.
X. Shi, L. Zhao, W. Wang, B. Zeng, L. Zhao, Y. Shan, M. Shen and K. Yang, Trans. Mater. Heat Treat., 2013, vol. 34, No. 7, pp. 47-52
13. Y. Liu, W. Zhang, Q. Tong and L. Wang, ISIJ International, 2014, vol. 54, pp. 1920 - 26.
14. Y. Liu, W. Zhang, Q. Tong, Q. Sun, International Journal of Iron and Steel Research, 2016, vol. 23, pp. 1316 - 22.
15. F. Zhao, C. L. Zhang, Q. Xiu, Y. Tan, S. Zhang, and Y. Z. Liu, Materials Science Forum, 2015, vol. 817, pp. 132 - 36.
16. F. Zhao, C. L. Zhang, Y. Z. Liu, Arch. Metall. Mater., 2016, vol. 61, pp. 1715 – 22.
N. Birks, Decarburization, Philadelphia: ISI Publication, 1969, Pp. 1-12
18. N. Birks and W. Jackson, Journal of Iron and Steel Institute, 1970, vol. 208, pp. 81-85.
N. Birks, G. Meier and F. S. Pettit, Introduction to the High-Temperature Oxidation of Metals, (1 ed), Edward Arnold, London, 1983, Pp 175-84
N. Birks, G. Meier and F. S. Pettit, Introduction to the High-Temperature Oxidation of Metals, (2 Ed), Cambridge University Press, Cambridge, 2006, pp. 151-62
R.Y. Chen, W.Y.D. Yuen (2008). In: Gao W, Li Z (eds) Developments in High-Temperature Corrosion and Protection of Materials, Woodhead Publishing Limited, Cambridge, pp. 192-252, (2008)
21. R. Y. Chen and W. Y. D. Yuen, ISIJ International, 2005, vol. 45, pp. 52-59.
22. R. Y. Chen and W. Y. D. Yuen, Metallurgical and Materials Transactions A, 2009, vol. 40A, pp. 3091-3107.
23. W. S. Cao, S. -L. Chen, F. Zhang, K. Wu, Y. Yang, Y.A. Chang, R. Schmid-Fetzer and W. A. Oates, Calphad, 2009, vol. 33, pp. 328-42.
PanFe. Thermodynamic database for Fe-based alloys, CompuTherm, LLC: Middleton WI 53562 (2019)
25. W. Jost: Diffusion in Solids, Liquids and Gases, Academic Press Inc., New York, 1960, pp. 69-71.
26. R. P. Smith, Transaction of the Metallurgical Society of AIME, 1962, vol. 224, pp. 105-11.
27. A. E. Lord, Jr. and D. N. Beshers, Acta Melallurgica, 1966, vol. 14, pp. 1659-72.
28. R. Y. Chen, Oxidation of Metals, 2018, vol. 89, pp. 1-31.
29. R. Collin, S. Gunnarson and D. Thulin, Journal of the Iron and Steel Institute, 1972, vol. 210, pp. 785 – 89.
30. R. M. Asimov, Transaction of the Metallurgical Society of AIME, 1964, vol. 230, pp. 611-13.
RP Smith (1953) Acta Metall, 1:578 – 87
C. Wells, W. Batz and R. F. Mehl, Transactions AIME, 1950, vol 188, Journal of Metals, Transactions, (1951), vol. 2, pp. 553 – 60
33. G. Parrish and G. S. Harper, Production Gas Carburising, Pergamon Press, Oxford, New York, 1985, pp. 114- 16.
M.A. Krishtal, Diffusion processes in iron alloys, Israel Program for Scientific Translations, Jerusalem, pp. 90–133 (1970)
35. S. S. Babu and H. K. D. H. Bhadeshia, Journal of Materials Science Letters, 1995, vol. 14, pp. 314-16.
36. S.-J. Lee, D. K. Matlock and C. J. Von Tyne, ISIJ International, 2011, vol. 51, pp. 1903-11.
37. S.-J. Lee, D. K. Matlock and C. J. Van Tyne, Scripta Materialia, 2011, vol. 64, pp. 805-808.
38. S. K. Roy, H. J. Grabke and W. W. Düsseldorf, Arch. Eisenhüttenwess, 1980, vol. 51, pp. 91-96.
H. K. D. H. Bhadeshia, Metall. Mater. Trans. A, 2010, vol. 41A, pp. 1605 – 15
L. S. Darken, Transactions of AIME, 1949, vol. 180, pp. 430-38
M. E. Blanter, Diffusion processes in austenite and hardenability of alloyed steels, Thesis, Moskovski Institut Stali, Moscow, (1949)
41. J. K. Stanley, Metal Transactions, 1949, vol. 185, pp. 752-61.
42. C. G. Homan, Acta Metallurgica, 1964, vol. 12, pp. 1071 – 79.
43. A. A. Vasilyev and P. A. Golikov, Materials Physics and Mechaics, 2018, vol. 39, pp. 111-19.
44. R. B. Mclellan and P. Chraska, Materials Science and Engineering, 1971, vol. 7, pp. 305-17.
D. E. Jiang and E. A. Carter, Physical Review B, 2003, vol. 67, pp. 214103
46. A. Pelton, P. Koukkari, R. Pajarre, and G. Eriksson, Journal Chemical Thermodynamics, 2014, vol. 72, pp. 16-22.
47. M. Hillert, Phase Equilibria, Phase Diagrams and Phase Transformation – Their Thermodynamic Basis, (second ed.), Cambridge University Press, Cambridge UK, 2008, pp. 311 – 15.
48. J. Takada and M. Adachi, Journal of Materials Science 1986, vol. 21, pp. 2133-37.
49. H. Li, J. Zhang and D. J. Young, Materials at High Temperatures, 2011, vol. 28, pp. 297 – 301.
50. H. Li, J. Zhang and D. J. Young, Corrosion Science, 2012, vol. 54, pp. 127-38.
K. Kusabiraki, R. Watanabe, T. Ikehata, M. Takeda, T. Onishi, X. Guo, H. Anada, Tetsu-to-Hagane, 2007, vol. 93, pp. 379–85
K. Kusabiraki, R. Watanabe, T. Ikehata, M. Takeda, T. Onishi, X. Guo and H. Anada, ISIJ International, 2007, vol. 47, pp. 1329-34
52. F. D. Richardson and J. H. E. Jeffes, Journal of Iron Steel Institute, 1948, vol. 160, pp. 261-70.
53. O. Kubaschewski and C. B. Alcock, Metallurgical Thermochemistry, Fifth Edition, Pergamon Press, Oxford, 1979, pp. 379.
54. J. A. Lobo and G. H. Gaiger, Metallurgical Transactions A, 1976, vol. 7A, pp. 1347-57.
Y.R. Chen, X. Xu, and Y. Liu, Oxid. Metals 2020, vol. 93, pp. 105–29.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted October 7, 2019.
Appendix I: Determination of the Carbon Permeability Through the Ferrite Layer[30]:
Appendix I: Determination of the Carbon Permeability Through the Ferrite Layer[30]:
In determining the equilibrium carbon concentration at the scale–steel interface, it is assumed that the dissolved carbon in steel can react with wustite, forming either CO and CO2via the following reactions:
and the overall reaction of these reactions becomes:
Using the data given by Richardson and Jeffes[56] and quoted by Kubaschewski and Alcock,[57] the standard Gibbs free energy of formation for Reaction (A3) is given by
When the reaction (A3) reaches equilibrium,
The \( \frac{{P_{\text{CO}} }}{{P_{{{\text{CO}}_{2} }} }} \) thus obtained can be used to determine the equilibrium carbon activity at the interface assuming \( P_{\text{CO}} \) + \( P_{{{\text{CO}}_{2} }} \) = 1 atm from the following reaction:
The standard Gibbs free energy of formation for this reaction is given by,[56,57]
where \( a_{c} \) is the equilibrium activity of carbon at the scale-steel interface with graphite being its standard state. From Eq. [A7], we obtain,
After the equilibrium carbon activity at the interface is determined, the corresponding carbon concentration in the steel at the scale-steel interface can be calculated using the known relationships between carbon activities and carbon concentrations. For dissolved carbon in α-Fe, the relationship to express the activity coefficient of carbon in ferrite for carbon steel, \( \varUpsilon_{\text{C}} \left( {\text{ferrite}} \right) \), is given by,[58]
where \( X_{\text{C}} \) is the equilibrium molar fraction of carbon in ferrite at the scale-steel interface, which can be converted to carbon concentration in weight percent using the following equation,
When a ferrite layer formed on the steel surface and the alloy concentrations are constants across the ferrite layer, the difference in the carbon concentration between two interfaces of the ferrite layer, \( \Delta C_{\text{C}}^{\alpha } \), provides a driving force for carbon to diffuse through the ferrite layer.
When the ferrite layer is thin, the carbon distribution in it can be approximated as having a linear concentration gradient and carbon diffusion through this layer can be described using the simplified Fick’s first law:
where \( J_{\text{C}}^{\alpha } \) = the diffusion flux of carbon from the interface between the ferrite layer and the bulk of steel towards the scale-steel interface; \( D_{\text{C}}^{\alpha } \) = the diffusion coefficient of carbon in ferrite; \( C_{{{\text{C}} {\text{in}} \alpha }}^{\alpha - \gamma } \) = the carbon concentration on the ferrite side at the interface between the ferrite layer and the bulk of steel in wt pct ; \( C_{{{\text{C in}} \alpha }}^{{\alpha - {\text{FeO}}}} \)= the carbon concentration in ferrite at the steel-scale interface in wt pct ; \( A \) = a constant used to convert the concentration of carbon from wt pct to mole/cm3; \( M \) = the thickness of the ferrite layer.
From Eq. [A11], we can see that for a certain thickness of the ferrite layer, \( X \), the rate of carbon diffusion is determined by the product of carbon diffusivity \( D_{\text{C}}^{\alpha } \) and the carbon concentration difference between the two interfaces of the ferrite layer,\( \cdot \Delta C_{\text{C}}^{\alpha } = C_{{{\text{C in}} \alpha }}^{\alpha - \gamma } - C_{{{\text{C}} {\text{in}} \alpha }}^{{\alpha - {\text{FeO}}}} \). Following the approach used by Smith,[28] the following product was defined as the permeability or relative permeability,[59] (\( P_{\text{C}}^{\alpha } \)) of carbon through the ferrite layer,
Substitution of Eq. [A12] in Eq. [A11] yields,
It can be seen that a greater permeability immediately leads to a greater carbon flux for a given ferrite layer thickness.
Strictly speaking, the alloying effect, particularly the effect of Si, should be considered in calculating the carbon concentration from the carbon activity data obtained from Eq. [A8]. However, as internal oxidation was inevitably observed, it was assumed that the dissolved alloying components (Si, Mn and Cr) had reacted with dissolved oxygen having diffused into the steel and therefore, the alloying effect from the remaining dissolved alloying components at the FeO-steel interface was considered to be negligible.
Rights and permissions
About this article
Cite this article
Chen, Y.R., Zhang, F. & Liu, Y. Decarburization of 60Si2MnA in 20 Pct H2O-N2 at 700 °C to 900 °C. Metall Mater Trans A 51, 1808–1821 (2020). https://doi.org/10.1007/s11661-020-05644-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-020-05644-0