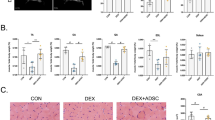
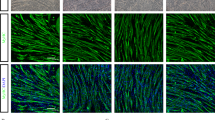
Abstract
The therapeutic potential of mesenchymal stem cell-conditioned medium (MSC-CM) has been reported with various types of disease models. Here, we examine the therapeutic effect of umbilical cord MSC-CM (UCMSC-CM) on muscle-related disease, using a dexamethasone (Dex)-induced muscle atrophy in vitro model. The expressions of muscle atrophy-related proteins (MuRF-1 and MAFbx) and muscle-specific proteins (desmin and myogenin) were evaluated by Western blot analysis. The level of production of reactive oxygen species (ROS) was determined using a 2′,7′-dichlorofluorescein diacetate (DCFDA) dye assay. The expression of antioxidant enzymes (copper/zinc-superoxide dismutase (Cu/Zn-SOD), manganese superoxide dismutase (MnSOD), glutathione peroxidase-1 (GPx-1), and catalase (CAT)) was verified by reverse transcription polymerase chain reaction (RT-PCR). When L6 cells were exposed to Dex, the expression of muscle atrophy-related proteins was increased by 50–70%, and the expression of muscle-specific proteins was in turn decreased by 23–40%. Conversely, when the L6 cells were co-treated with UCMSC-CM and Dex, the expression of muscle atrophy-related proteins was reduced in a UCMSC-CM dose-dependent manner and the expression of muscle-specific proteins was restored to near-normal levels. Moreover, ROS generation was effectively suppressed and the expression of antioxidant enzymes was recovered to a normal degree. These data imply that UCMSC-CM clearly has the potential to prevent muscle atrophy. Thus, our present study offers fundamental data on the potential treatment of muscle-related disease using UCMSC-CM.





Similar content being viewed by others
References
Angoulvant D, Ivanes F, Ferrera R, Matthews PG, Nataf S, Ovize M (2011) Mesenchymal stem cell conditioned media attenuates in vitro and ex vivo myocardial reperfusion injury. J Heart Lung Transplant 30:95–102
Bonaldo P, Sandri M (2013) Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech 6:25–39
Cantinieaux D, Quertainmont R, Blacher S, Rossi L, Wanet T, Noël A, Brook G, Schoenen J, Franzen R (2013) Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PLoS One 8, e69515
Chen L, Tredget EE, Wu PY, Wu Y (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3, e1886
Choi MY, Park KH (2014) Establishment of endogenous human tympanic membrane-derived somatic stem cells for stem cell therapy. In Vitro Cell Dev Biol Anim 50(8):747–755
Corti S, Nizzardo M, Nardini M, Donadoni C, Salani S, Ronchi D, Simone C, Falcone M, Papadimitriou D, Locatelli F (2010) Embryonic stem cell-derived neural stem cells improve spinal muscular atrophy phenotype in mice. Brain 133:465–481
Friedman R, Betancur M, Boissel L, Tuncer H, Cetrulo C, Klingemann H (2007) Umbilical cord mesenchymal stem cells: adjuvants for human cell transplantation. Biol Blood Marrow Transplant 13:1477–1486
Gibson J, Smith K, Rennie M (1988) Prevention of disuse muscle atrophy by means of electrical stimulation: maintenance of protein synthesis. Lancet 332:767–770
Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS (2006) Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 20:661–669
Hamrick MW (2012) The skeletal muscle secretome: an emerging player in muscle-bone crosstalk. BoneKey Rep 1(60):1–5
Hussain SP, Amstad P, He P, Robles A, Lupold S, Kaneko I, Ichimiya M, Sengupta S, Mechanic L, Okamura S (2004) p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res 64:2350–2356
Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA (2001) Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 107:1395–1402
Kern S, Eichler H, Stoeve J, Klüter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294–1301
Kim HO, Choi S-M, Kim H-S (2013a) Mesenchymal stem cell-derived secretome and microvesicles as a cell-free therapeutics for neurodegenerative disorders. Tissue Eng Regen Med 10:93–101
Kim HY, Kim H, Oh KW, Oh SI, Koh SH, Baik W, Noh MY, Kim KS, Kim SH (2014a) Biological markers of mesenchymal stromal cells as predictors of response to autologous stem cell transplantation in patients with amyotrophic lateral sclerosis: an investigator‐initiated trial and in vivo study. Stem Cells 32:2724–2731
Kim J, Lee JH, Yeo SM, Chung HM, Chae JI (2014b) Stem cell recruitment factors secreted from cord blood-derived stem cells that are not secreted from mature endothelial cells enhance wound healing. In Vitro Cell Dev Biol Anim 50(2):146–154
Kim MJ, Kim YM, Kim Z-H, Heo S-H, Kim S-M, Hwang J-W, Chang W-J, Baek MJ, Choi YS (2015) Mesenchymal stem cells suppress muscle atrophy induced by hindlimb suspension. J Stem Cell Res Ther 5:2
Kim S-M, Moon SH, Lee Y, Kim GJ, Chung HM, Choi Y-S (2013b) Alternative xeno-free biomaterials derived from human umbilical cord for the self-renewal ex-vivo expansion of mesenchymal stem cells. Stem Cells Dev 22:3025–3038
Kim S-Y, Lee J-H, Kim HJ, Park MK, Huh JW, Ro JY, Oh Y-M, Lee S-D, Lee Y-S (2012) Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. Am J Physiol Lung Cell Mol Physiol 302:L891–L908
Kim W-S, Park B-S, Kim H-K, Park J-S, Kim K-J, Choi J-S, Chung S-J, Kim D-D, Sung J-H (2008) Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci 49:133–142
Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F (2011) Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells 29:11–19
Kollias HD, McDermott JC (2008) Transforming growth factor-β and myostatin signaling in skeletal muscle. J Appl Physiol 104:579–587
Kouidi E, Albani M, Natsis K, Megalopoulos A, Gigis P, Guiba-Tziampiri O, Tourkantonis A, Deligiannis A (1998) The effects of exercise training on muscle atrophy in haemodialysis patients. Nephrol Dial Transplant 13:685–699
Lee S-J, McPherron AC (1999) Myostatin and the control of skeletal muscle mass: commentary. Curr Opin Genet Dev 9:604–607
Leeuwenburgh C (2003) Role of apoptosis in sarcopenia. J Gerontol A Biol Sci Med Sci 58:999–1001
Li S, Yan T, Yang J-Q, Oberley TD, Oberley LW (2000) The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Cancer Res 60:3927–3939
Moghadasali R, Mutsaers HA, Azarnia M, Aghdami N, Baharvand H, Torensma R, Wilmer MJ, Masereeuw R (2013) Mesenchymal stem cell-conditioned medium accelerates regeneration of human renal proximal tubule epithelial cells after gentamicin toxicity. Exp Toxicol Pathol 65:595–600
Primeau AJ, Adhihetty PJ, Hood DA (2002) Apoptosis in heart and skeletal muscle. Can J Appl Physiol 27:349–395
Ranganath SH, Levy O, Inamdar MS, Karp JM (2012) Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 10:244–258
Rantanen J, Hurme T, Lukka R, Heino J, Kalimo H (1995) Satellite cell proliferation and the expression of myogenin and desmin in regenerating skeletal muscle: evidence for two different populations of satellite cells. Lab Invest 72:341–347
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117:399–412
Schakman O, Kalista S, Barbe C, Loumaye A, Thissen J (2013) Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol 45:2163–2172
Senf SM, Dodd SL, McClung JM, Judge AR (2008) Hsp70 overexpression inhibits NF-κB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J 22:3836–3845
Stecklum M, Wulf-Goldenberg A, Purfürst B, Siegert A, Keil M, Eckert K, Fichtner I (2015) Cell differentiation mediated by co-culture of human umbilical cord blood stem cells with murine hepatic cells. In Vitro Cell Dev Biol Anim 51(2):183–191
Yohn DC, Miles GB, Rafuse VF, Brownstone RM (2008) Transplanted mouse embryonic stem-cell-derived motoneurons form functional motor units and reduce muscle atrophy. J Neurosci 28:12409–12418
Acknowledgments
This work was supported by the Space Core Technology Development Program funded by the Ministry of Education Science and Technology (MEST), Korea (Project No.: 2011-0030754), for which the authors are grateful.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Editor: Tetsuji Okamoto
Chan-Mi Park and Mi Jin Kim contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
(DOCX 184 kb)
Rights and permissions
About this article
Cite this article
Park, CM., Kim, M.J., Kim, SM. et al. Umbilical cord mesenchymal stem cell-conditioned media prevent muscle atrophy by suppressing muscle atrophy-related proteins and ROS generation. In Vitro Cell.Dev.Biol.-Animal 52, 68–76 (2016). https://doi.org/10.1007/s11626-015-9948-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-015-9948-1