Abstract
Purpose
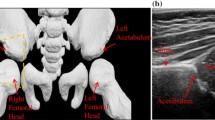
Automatic segmentation of anatomical structures and lesions from medical ultrasound images is a formidable challenge in medical imaging due to image noise, blur and artifacts. In this paper we present a segmentation technique with features highly suited to use in noisy 3D ultrasound volumes and demonstrate its use in modeling bone, specifically the acetabulum in infant hips. Quantification of the acetabular shape is crucial in diagnosing developmental dysplasia of the hip (DDH), a common condition associated with hip dislocation and premature osteoarthritis if not treated. The well-established Graf technique for DDH diagnosis has been criticized for high inter-observer and inter-scan variability. In our earlier work we have introduced a more reliable instability metric based on 3D ultrasound data. Visualizing and interpreting the acetabular shape from noisy 3D ultrasound volumes has been one of the major roadblocks in using 3D ultrasound as diagnostic tool for DDH. For this study we developed a semiautomated segmentation technique to rapidly generate 3D acetabular surface models and classified the acetabulum based on acetabular contact angle (ACA) derived from the models. We tested the feasibility and reliability of the technique compared with manual segmentation.
Methods
The proposed segmentation algorithm is based on graph search. We formulate segmentation of the acetabulum as an optimal path finding problem on an undirected weighted graph. Slice contours are defined as the optimal path passing through a set of user-defined seed points in the graph, and it can be found using dynamic programming techniques (in this case Dijkstra’s algorithm). Slice contours are then interpolated over the 3D volume to generate the surface model. A three-dimensional ACA was calculated using normal vectors of the surface model.
Results
The algorithm was tested over an extensive dataset of 51 infant ultrasound hip volumes obtained from 42 subjects with normal to dysplastic hips. The contours generated by the segmentation algorithm closely matched with those obtained from manual segmentation. The average RMS errors between the semiautomated and manual segmentation for the 51 volumes were 0.28 mm/1.1 voxel (with 2 node points) and 0.24 mm/0.9 voxel (with 3 node points). The semiautomatic algorithm gave visually acceptable results on images with moderate levels of noise and was able to trace the boundary of the acetabulum even in the presence of significant shadowing. Semiautomatic contouring was also faster than manual segmentation at 37 versus 56 s per scan. It also improved the repeatability of the ACA calculation with inter-observer and intra-observer variability of \(1.4 \pm 0.9\) degree and \(1.4 \pm 1.0\) degree.
Conclusion
The semiautomatic segmentation technique proposed in this work offers a fast and reliable method to delineate the contours of the acetabulum from 3D ultrasound volumes of the hip. Since the technique does not rely upon contour evolution, it is less susceptible than other methods to the frequent missing or incomplete boundaries and noise artifacts common in ultrasound images. ACA derived from the segmented 3D surface was able to accurately classify the acetabulum under the categories normal, borderline and dysplastic. The semiautomatic technique makes it easier to segment the volume and reduces the inter-observer and intra-observer variation in ACA calculation compared with manual segmentation. The method can be applied to any structure with an echogenic boundary on ultrasound (such as a ventricle, blood vessel, organ or tumor), or even to structures with a bright border on computed tomography or magnetic resonance imaging.














Similar content being viewed by others
References
Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI (2001) Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987–99. 83:579–86
Graf R (1984) Fundamentals of sonographic diagnosis of infant hip dysplasia. J Pediatr Orthop 4(6):735–740
Jaremko JL, Mabee M, Swami VG, Jamieson L, Chow K, Thompson RB (2014) Potential for change in US diagnosis of hip dysplasia solely caused by changes in probe orientation: patterns of alpha-angle variation revealed by using three-dimensional US. Radiology 273(3):870–878
Mabee M, Dulai S, Thompson RB, Jaremko JL (2014) Reproducibility of Acetabular landmarks and a standardized coordinate system obtained from 3D hip ultrasound. Ultrason Imaging 0161734614558278
Noble JA, Boukerroui D (2006) Ultrasound image segmentation: a survey. IEEE Trans Med Imaging 25(8):987–1010
Ehrhardt J, Handels H, Malina T, Strathmann B, Plötz W, Pöppl Siegfried J (2001) Atlas-based segmentation of bone structures to support the virtual planning of hip operations. Int J Med Inf 64(2):439–447
Zoroofi RA, Sato Y, Sasama T, Nishii T, Sugano N, Yonenobu K, Yoshikawa H, Ochi T, Tamura S (2003) Automated segmentation of acetabulum and femoral head from 3-D CT images. Inf Technol Biomed, IEEE Trans 7(4):329–343
Boukala N, Favier E, Laget B, Radeva P (2004) Active shape model based segmentation of bone structures in hip radiographs. In Industrial Technology, 2004. IEEE ICIT’04. 2004 IEEE International Conference on, 3:1682–1687. IEEE
Yokota F, Okada T, Takao M, Sugano N, Tada Y, Sato Y (2009) Automated segmentation of the femur and pelvis from 3D CT data of diseased hip using hierarchical statistical shape model of joint structure. In Medical image computing and computer-assisted intervention-MICCAI 2009. Springer, Berlin, pp 811–818
Kainmueller D, Lamecker H, Zachow S, Hege H-C (2009) An articulated statistical shape model for accurate hip joint segmentation. In Engineering in Medicine and Biology Society, 2009. EMBC 2009. Annual International Conference of the IEEE, pp. 6345–6351. IEEE
Zoroofi RA, Sato Y, Nishii T, Sugano N, Yoshikawa H, Tamura Shinichi (2004) Automated segmentation of necrotic femoral head from 3D MR data. Comput Med Imaging Gr 28(5):267–278
Gilles B, Moccozet L, Magnenat-Thalmann N (2006) Anatomical modelling of the musculoskeletal system from MRI. Medical Image Computing and Computer-Assisted Intervention-MICCAI 2006. Springer, Berlin, pp 289–296
de Luis-Garcia R, Alberola-López C (2006) Parametric 3D hip joint segmentation for the diagnosis of developmental dysplasia. 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS)
Mortensen EN, Barrett WA (1998) Interactive segmentation with intelligent scissors. Gr Models Image Process 60(5):349–384
Mortensen EN, Barrett WA (1995) Intelligent scissors for image composition. Proceedings of the 22nd annual conference on Computer graphics and interactive techniques. ACM
Barrett WA, Mortensen EN (1997) Interactive live-wire boundary extraction. Med Image Analysis 1(4):331–341
Li K, Xiaodong W, Chen DZ, Sonka M (2006) Optimal surface segmentation in volumetric images-a graph-theoretic approach. Pattern Anal Machine Intell, IEEE Trans 28(1):119–134
Hamarneh G, Yang J, McIntosh C, Langille M (2005) 3D live-wire-based semi-automatic segmentation of medical images. In Medical imaging, pp 1597–1603. International Society for Optics and Photonics
Falcao AX, Udupa JK (1997) Segmentation of 3D objects using live wire. In Medical imaging 1997, pp 228–235. International Society for Optics and Photonics
Falcão AX, Udupa JK, Miyazawa FK (2000) An ultra-fast user-steered image segmentation paradigm: live wire on the fly. Med Imaging, IEEE Trans 19(1):55–62
Mishra A, Wong A, Zhang W, Clausi D, Fieguth P (2008) Improved interactive medical image segmentation using enhanced intelligent scissors (eis). In Engineering in Medicine and Biology Society, 2008. EMBS 2008. 30th Annual International Conference of the IEEE, pp 3083–3086. IEEE
Boykov YY, Jolly MP (2001) Interactive graph cuts for optimal boundary & region segmentation of objects in ND images. Proceedings of the Eighth IEEE International Conference on Computer Vision, (ICCV). Vol. 1
Grady L (2006) Random walks for image segmentation. IEEE Trans Pattern Anal Mach Intell 28(11):1768–1783
Punithakumar K, Yuan J, Ben Ayed I, Li S, Boykov Y (2012) A convex max-flow approach to distribution-based figure-ground separation. SIAM J Imaging Sci 5(4):1333–1354
Dijkstra EW (1959) A note on two problems in connexion with graphs. Numerische Mathematik 1.1: 269–271
Acknowledgments
The authors wish to thank Servier Canada, Radiologic Society of North America (RSNA) Research Seed Grant and CIHR Institute of Human Development, Child and Youth Health (IHDCYH), Grant NI15-004 for the research funding which supported this work. We also thank Dr Pierre Boulanger (Scientific Director, Servier Virtual Cardiac Centre, University of Alberta) for his insight and expertise that greatly helped in this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors Abhilash Rakkunedeth Hareendranathan, Myles Mabee, Kumaradevan Punithakumar, Michelle Noga and Jacob L. Jaremko declare that they have no conflict of interest.
Ethical standard
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed consent
Informed consent was obtained from all patients for being included in the study.
Rights and permissions
About this article
Cite this article
Hareendranathan, A.R., Mabee, M., Punithakumar, K. et al. A technique for semiautomatic segmentation of echogenic structures in 3D ultrasound, applied to infant hip dysplasia. Int J CARS 11, 31–42 (2016). https://doi.org/10.1007/s11548-015-1239-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-015-1239-5