Abstract
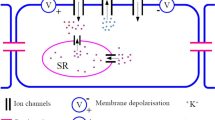
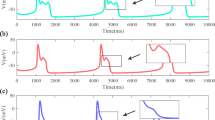
Evidence from experimental studies shows that oscillations due to electro-mechanical coupling can be generated spontaneously in smooth muscle cells. Such cellular dynamics are known as pacemaker dynamics. In this article, we address pacemaker dynamics associated with the interaction of \({\text {Ca}}^{2+}\) and \(\text {K}^+\) fluxes in the cell membrane of a smooth muscle cell. First we reduce a pacemaker model to a two-dimensional system equivalent to the reduced Morris–Lecar model and then perform a detailed numerical bifurcation analysis of the reduced model. Existing bifurcation analyses of the Morris–Lecar model concentrate on external applied current, whereas we focus on parameters that model the response of the cell to changes in transmural pressure. We reveal a transition between Type I and Type II excitabilities with no external current required. We also compute a two-parameter bifurcation diagram and show how the transition is explained by the bifurcation structure.












Similar content being viewed by others
References
Barnett W, Cymbalyuk G (2014) A codimension-2 bifurcation controlling endogenous bursting activity and pulse-triggered responses of a neuron model. PLoS ONE 9(1):e85451
Berra-Romani R, Blaustein MP, Matteson DR (2005) TTX-sensitive voltage-gated \(\text{ Na}^{+}\) channels are expressed in mesenteric artery smooth muscle cells. Am J Physiol Heart Circ Physiol 289:H137–H145
Bianchi D, Morin C, Badel P (2019) Computational modeling of arterial tissues: coupling multiscale homogenization strategies and finite element formulation. Comput Methods Biomech Biomed Eng 22(sup1):S22–S24
Bogdanov RI (1975) Versal deformations of a singular point of a vector field on the plane in the case of zero eigenvalues. Funct Anal Its Appl 9:144–145
Brading AF (2006) Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol 570(1):13–22
Casteels R, Kitamura K, Kuriyama H, Suzuki H (1977) Excitation-contraction coupling in the smooth muscle cells of the rabbit main pulmonary artery. J Physiol 271(1):63–79
Christini DJ, Stein KM, Markowitz SMS, Mittal Slotwiner DJ, Lerman BB (1999) The role of nonlinear dynamics in cardiac arrhythmia control. Heart Dis 1(4):190–200
Connor JA (1985) Neural pacemakers and rhythmicity. Ann Rev Physiol 47:17–28
Crook SM, Ermentrout GB, Bower JM (1998) Spike frequency adaptation affects the synchronization properties of networks of cortical oscillators. Neural Comput 10(4):837–854
Doedel EJ, Oldeman BE, Wang X, Zhang C (2012) AUTO-07P: Continuation and bifurcation software for ordinary differential equations. Tech. rep, Montreal
Droogmans G, Casteels R (1989) Electromechanical and pharmacomechanical coupling in vascular smooth muscle. In: Sperelakis N (ed) Physiology and pathophysiology of the heart. Developments in cardiovascular medicine. Springer, Boston, pp 813–824
Duan L, Lu Q (2006) Codimension-two bifurcation analysis on firing activities in Chay neuron model. Chaos Solitons Fractals 30(5):1172–1179
Duan L, Lu Q, Wang Q (2008) Two-parameter bifurcation analysis of firing activities in the Chay neuronal model. Neurocomputing 72(1–3):341–351
Ermentout B (1996) Type I membranes, phase resetting curves and sychrony. Neural Comput 8(5):979–1001
Ermentrout B (2002) Simulating, analyzing, and animating dynamical systems: a guide to XPPAUT for researchers and students. SIAM Press, Philadelphia
FitzHugh R (1961) Impulses and physiological states in theoretical model of nerve membrane. Biophys J 1:445–466
Gonzalez-Fernandez JM, Ermentrout B (1994) On the origin and dynamics of the vasomotion of small arteries. Math Biosci 119:127–167
González-Miranda JM (2012) Nonlinear dynamics of the membrane potential of a bursting pacemaker cell. Chaos 22(1):013123
González-Miranda JM (2014) Pacemaker dynamics in the full Morris–Lecar model. Commun Nonlinear Sci Numer Simul 19:3229–3241
Govaerts W, Sautois B (2005) The onset and extinction of neural spiking: a numerical bifurcation approach. J Comput Neurosci 18(3):265–274
Gu H (2013a) Biological experimental observations of an unnoticed Chaos as simulated by the hindmarsh-rose model. PLoS ONE 8(12):e81759
Gu H (2013b) Experimental observation of transition from chaotic bursting to chaotic spiking in a neural pacemaker. Chaos 23(2):023126
Haddock RE, Hirst GD, Hill CE (2002) Voltage independence of vasomotion in isolated irideal arterioles of the rat. J Physiol 540:219–229
Harder DR (1984) Pressure-dependent membrane depolarization in cat middle cerebral artery. Circ Res 55:197–202
Harder DR (1987) Pressure-induced myogenic activation of cat cerebral arteries is dependent on intact endothelium. Circ Res 60:102–107
He F, Hua L, Gao L (2015) A computational model for biomechanical effects of arterial compliance mismatch. Appl Bion Biomech 2015:213236
Hindmarsh JL, Rose RM (1984) A model of neuronal bursting using three coupled first order differential equations. Proc R Soc Lond B 221:87–102
Hodgkin AL (1948) The local electric changes associated with repetitive action in a non-medullated axon. J Physiol 107(2):165–181
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117(4):500–544
Humphrey JD, Wilson E (2003) A potential role of smooth muscle tone in early hypertension: a theoretical study. J Biomech 36(11):1595–1601
Izhikevich EM (2007) Dynamical systems in neuroscience: the geometry of excitability and bursting. MIT Press, Cambridge
Jia B (2018) Negative feedback mediated by fast Inhibitory autapse enhances neuronal oscillations near a Hopf bifurcation point. Int J Bifurc Chaos 28(2):1850030
Jia B, Gu H, Xue L (2017) A basic bifurcation structure from bursting to spiking of injured nerve fibers in a two-dimensional parameter space. Cogn Neurodyn 11:189–200
Koenigsberger M, Sauser R, Bé J, Meister J (2005) Role of the endothelium on arterial vasomotion. Biophys J 88:3845–3854
Kuznetsov YA (1995) Elements of applied bifurcation theory, 3rd edn. Springer, New York
Lamboley M, Schuster A, Bény JL, Meister JJ (2003) Recruitment of smooth muscle cells and arterial vasomotion. Am J Physiol 285:H562–H569
Liu C, Liu X, Liu S (2014) Bifurcation analysis of a Morris–Lecar neuron model. Biol Cybern 108(1):75–84
Llinas RR (1988) The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science 242(4886):1654–1664
Lusamvuku NA, Sercombe R, Aubineau P, Seylaz J (1979) Correlated electrical and mechanical responses of isolated rabbit pial arteries to some vasoactive drugs. Stroke 10(6):727–732
Ma J, Tang J (2015) A review for dynamics of collective behaviors of network of neurons. Sci China Technol Sci 58(12):2038–2045
Mahapatra C, Brain KL, Manchanda R (2018) A biophysically constrained computational model of the action potential of mouse urinary bladder smooth muscle. PLoS ONE 13(7):e0200712
Mclean MJ, Sperelakis N (1977) Electrophysiological recordings from spontaneously contracting reaggregates of cultured vascular smooth muscle cells from chick embryos. Exp Cell Res 104(2):309–318
Meier SR, Lancaster JL, Starobin JM (2015) Bursting regimes in a reaction–diffusion system with action potential-dependent equilibrium. PLoS ONE 10(3):1–25
Meiss JD (2007) Differential dynamical systems, 1st edn. SIAM, Philadelphia
Meister M, Wong RL, Baylor DA, Shatz CJ (1991) Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science 252(5008):939–943
Meyer JU, Lindbom L, Intaglietta M (1983) Pacemaker induced diameter oscillations at arteriolar bifurcations in skeletal muscle. Prog Appl Microcirc 12:264–269
Meyer JU, Borgstrom P, Intaglietta M (1988) Is vasomotion due to microvascular pacemaker cells? Prog Appl Mircocirc 15:41–48
Mondal A, Upadhyay RK, Mondal A, Sharma SK (2018) Dynamics of a modified excitable neuron model: diffusive instabilities and traveling wave solutions. Chaos 28(11):113104
Mondal A, Upadhyay RK, Ma J, Yadav BK, Sharma SK (2019) Bifurcation analysis and diverse firing activities of a modified excitable neuron model. Cogn Neurodyn 13(4):393–407
Morris C, Lecar H (1981) Voltage oscillations in the barnacle giant muscle fiber. Biophys J 35:193–213
Nagumo J, Arimoto S, Yoshizawa S (1962) An active pulse transmission line simulating nerve axon. Proc IRE 50(10):2061–2070
Osol GJ, Halpern W (1988) Spontaneous vasomotion in pressurized cerebral arteries from genetically hypertensive rats. Am J Physiol 254(1 Pt 2):H28–33
Pogátsa G (1994) Altered responsiveness of vascular smooth muscle to drugs in diabetes. In: Szekeres L, Papp JG (eds) Pharmacology of smooth muscle. Handbook of experimental pharmacology. Springer, Berlin, pp 693–712
Prescott SA, De Koninck Y, Sejnowski TJ (2008) Biophysical basis for three distinct dynamical mechanisms of action potential initiation. PLoS Comput Biol 4(10):1000198
Ramirez JM, Tryba AK, Peña F (2004) Pacemaker neurons and neuronal networks: an integrative view. Curr Opin Neurobiol 14(6):665–674
Ran K, Yang Z, Zhao Y, Wang X (2019) Transmural pressure drives proliferation of human arterial smooth muscle cells via mechanism associated with NADPH oxidase and Survivin. Microvasc Res 126:103905
Rinzel J, Ermentrout GB (1999) Analysis of neural excitability and oscillations. In: Koch C, Segev I 2nd (eds) Methods in neuronal modeling: from ions to network. MIT Press, London, pp 251–291
Sala F, Hernandez-Cruz A (1990) Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J 57:313–324
Savineau J, Marthan R (2000) Cytosolic calcium oscillations in smooth muscle cells. News Physiol Sci 15(1):50–55
Segal SS, Duling BR (1989) Conduction of vasomotor responses in arterioles: a role for cell-to-cell coupling? Am J Physiol 256(3):H838–H845
Shaikh MA, Wall DJN, David T (2011) Macro-scale phenomena of arterial coupled cells: a massively parallel simulation. J R Soc Interface 9:972–987
Storace M, Linaro D, Lange E (2008) The Hindmarsh–Rose neuron model: bifurcation analysis and piecewise-linear approximations. Chaos 18(3):033128
Strogatz HS (1994) Nonlinear dynamics and chaos: with applications to physics, biology, chemistry and engineering, 1st edn. Perseus Books, Massachusetts
Sui G, Wu C, Fry C (2003) A description of Ca2+ channels in human detrusor smooth muscle. BJU Int 92(4):476–482
Takens F (1974) Singularities of vector fields. Publ Math IHES 43:47–100
Thorne B, Hayenga H, Humphrey J, Peirce S (2011) Toward a multi-scale computational model of arterial adaptation in hypertension: verification of a multi-cell agent based model. Front Physiol 2:20
Tsumoto K, Kitajima H, Yoshinaga T, Aihara K, Kawakami H (2006) Bifurcations in Morris–Lecar neuron model. Neurocomputing 69(4–6):293–316
Ulyanova AV, Shirokov RE (2018) Voltage-dependent inward currents in smooth muscle cells of skeletal muscle arterioles. PLoS ONE 13(4):e0194980
Verma P, Kienle A, Flockerzi D, Ramkrishna D (2020) Using bifurcation theory for exploring pain. Ind Eng Chem Res 59(6):2524–2535
Vreeswijk CV, Hansel D (2001) Patterns of synchrony in neural networks with spike adaptation. Neural Comput 13(5):959–992
Xie RG, Zheng DW, Xing JL, Zhang XJ, Song Y, Xie YB, Kuang F, Dong H, You SW, Xu H, Hu SJ (2011) Blockade of persistent sodium currents contributes to the riluzole-induced inhibition of spontaneous activity and oscillations in injured DRG neurons. PLoS ONE 6(4):e18681
Zhao Z, Gu H (2017) Transitions between classes of neuronal excitability and bifurcations induced by autapse. Sci Rep 7(1):6760
Acknowledgements
We thank Prof. Hinke M. Osinga (University of Auckland, New Zealand) for the support provided and useful discussion.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fatoyinbo, H.O., Brown, R.G., Simpson, D.J.W. et al. Numerical Bifurcation Analysis of Pacemaker Dynamics in a Model of Smooth Muscle Cells. Bull Math Biol 82, 95 (2020). https://doi.org/10.1007/s11538-020-00771-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-020-00771-6
Keywords
- Smooth muscle cells
- Electro-mechanical coupling
- Pacemaker dynamics
- Morris–Lecar
- Saddle-node on an invariant circle bifurcation
- Type I and II excitability