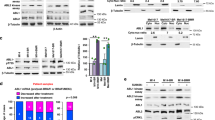
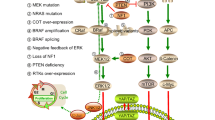
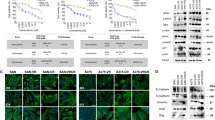
Abstract
One of the most frequently found mutations in human melanomas is in the B-raf gene, making its protein BRAF a key target for therapy. However, in patients treated with BRAF inhibitor (BRAFi), although the response is very good at first, relapse occurs within 6 months, on the average. In order to overcome this drug resistance to BRAFi, various combinations of BRAFi with other drugs have been explored, and some are being applied clinically, such as a combination of BRAF and MEK inhibitors. Experimental data for melanoma in mice show that under continuous treatment with BRAFi, the pro-cancer MDSCs and chemokine CCL2 initially decrease but eventually increase to above their original level, while the anticancer T cells continuously decrease. In this paper, we develop a mathematical model that explains these experimental results. The model is used to explore the efficacy of combinations of BRAFi with anti-CCL2, anti-PD-1 and anti-CTLA-4, with the aim of eliminating or reducing drug resistance to BRAFi.









Similar content being viewed by others
References
Abcam (1998–2019) Anti-MCP1 antibody (ab9669). https://www.abcam.com/mcp1-antibody-ab9669.html
Abcam (1998–2019) Anti-PD1 antibody (ab89828). https://www.abcam.com/pd1-antibody-ab89828.html
Abcam (1998–2019) Recombinant Anti-CTLA4 antibody [EPR1476] (ab134090). https://www.abcam.com/ctla4-antibody-epr1476-ab134090.html
Ascierto PA, Kirkwood JM, Grob JJ, Simeone E, Grimaldi AM, Maio M et al (2012) The role of BRAF v600 mutation in melanoma. J Transl Med 10(85):1–9
Beatty GL, Gladney WL (2015) Immune escape mechanisms as a guide for cancer immunotherapy. Oncologist 21(4):687–692
Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH et al (2010) Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 28(19):3167–3175
Butte MJ, Pena-Cruz V, Kim MJ, Freeman GJ, Sharpe AH (2008) Interaction of human PD-L1 and B7–1. Mol Immunol 45(13):3567–3572
Chang C, Liao JC, Kuo L (2001) Macrophage arginase promotes tumor cell growth and suppresses nitric oxide-mediated tumor cytotoxicity. Cancer research 61(3):1100–1106
Chen D, Roda JM, Marsh CB, Eubank TD, Friedman A (2012) Hypoxia inducible factors-mediated inhibition of cancer by GM-CSF: a mathematical model. Bull Math Biol 74(11):2752–2777
Cree IA, Charlton P (2017) Molecular chess? Hallmarks of anti-cancer drug resistance. BMC Cancer 17(10):1–8
D’Acunto B (2004) Computational methods for PDE in mechanics, series on advances in mathematics for applied sciences. World Scientific, Singapore, p 67
Díaz-Martinez M, Benito-Jardon L, Teixido J (2018) New insights in melanoma resistance to BRAF inhibitors: a role for microRNAs. Oncotarget 9(83):35374–35375
Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G (2013) Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-Cell rejection function in tumors. Cancer Res 73(12):3591–3603
Durgeau A, Virk Y, Corgnac S, Mami-Chouaib F (2018) Recent advances in targeting CD8 T-cell immunity for more effective cancer immunotherapy. Front Immunol 9(14):1–14
Eubank TD, Roberts RD, Khan M, Curry JM, Nuovo GJ, Kuppusamy P et al (2009) Granulocyte macrophage colony-stimulating factor inhibits breast cancer growth and metastasis by invoking an anti-angiogenic program in tumor-educated macrophages. Cancer Res 69(5):2133–2140
For Biotechnology Information NNC. Artesunate. Open Chem Database (2018);65664. https://pubchem.ncbi.nlm.nih.gov/compound/Artesunate#section=Top. Accessed 30 Mar 2019
Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR et al (2013) BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res 19(5):1225–1231
Friedman A, Hao W (2018) The role of exosomes in pancreatic cancer microenvironment. Bull Math Biol 80(5):1111–1133
Gaffney EA (2004) The application of mathematical modelling to aspects of adjuvant chemotherapy scheduling. J Math Biol 48(4):375–422
Gehad A, Lichtman M, Schmults C, Teague JE, Calarese A, Jiang Y et al (2012) Nitric oxide–producing myeloid-derived suppressor cells inhibit vascular E-selectin expression in human squamous cell carcinomas. J Investig Dermatol 132(11):2642–2651
Gillet JP, Gottesman MM (2009) Mechanisms of multidrug resistance in cancer. Meth Mol Biol Multi-Drug Res Cancer 596:47–76
Hagen B, Trinh VA (2014) Managing side effects of vemurafenib therapy for advanced melanoma. J Adv Pract Oncol 5:400–410
Hao W, Crouser ED, Friedman A (2014) Mathematical model of sarcoidosis. PNAS 111(45):16065–16070
Hao W, Schlesinger LS, Friedman A (2016) Modeling granuloma in response to infection in the lung. PLoS ONE 11(3):1–26
Hao W, Komar HM, Hart PA, Conwell DL, Lesinski GB, Friedman A (2017) Mathematical model of chronic pancreatitis. PNAS 114(19):5011–5016
Harpaz Y, Gerstein M, Chothia C (1994) Volume changes on protein folding. Structure 2(7):641–649
Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E (2015) PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res 43:D512–D520
Ilieva KM, Correa I, Josephs DH, Karagiannis P, Egbuniwe IU, Cafferkey MJ et al (2014) Effects of BRAF mutations and BRAF inhibition on immune responses to melanoma. Mol Cancer Ther 13(12):2769–2783
Jafarzadeh A, Minaee K, Farsinejad A, Nemati M, Khosravimashizi A, Daneshvar H et al (2015) Evaluation of the circulating levels of IL-12 and IL-33 in patients with breast cancer: influences of the tumor stages and cytokine gene polymorphisms. Iran J Basic Med Sci 18(12):1189–1198
Janco JMT, Lamichhane P, Karyampudi L, Knutson KL (2015) Tumor-infiltrating dendritic cells in cancer pathogenesis. J Immunol 194(7):2985–2991
Jobe NP, Rösel D, Dvorankova B, Kodet O, Lacina L, Mateu R et al (2016) Simultaneous blocking of IL-6 and IL-8 is sufficient to fully inhibit CAF-induced human melanoma cell invasiveness. Histochem Cell Biol 146(2):205–217
Kakadia S, Yarlagadda N, Awad R, Kundranda M, Niu J, Naraev B et al (2018) Mechanisms of resistance to BRAF and MEK inhibitors and clinical updates of US Food and Drug Administration-approved targeted therapy in advanced melanoma. Onco Targets Ther 11:7095–7107
Kawakami Y, Yaguchi T, Sumimoto H, Kudo-Saito C, Iwata-Kajihara T, Nakamura S et al (2013) Improvement of cancer immunotherapy by combining molecular targeted therapy. Front Oncol 3(136):1–7
Khunweeraphong N, Kuchler TSK (2017) The structure of the human ABC transporter ABCG2 reveals a novel mechanism for drug extrusion. Sci Rep 7(13767):1–15
Kim Y, Lawler S, Nowicki MO, Chiocca EA, Friedman A (2009) A mathematical model for pattern formation of glioma cells outside the tumor spheroid core. J Theor Biol 260(3):359–371
Kim Y, Wallace J, Li F, Ostrowski M, Friedman A (2010) Transformed epithelial cells and fibroblasts/myofibroblasts interaction in breast tumor: a mathematical model and experiments. J Theol Biol 61(3):401–421
Kirschner DE (2007–2008) Uncertainty and sensitivity functions and implementation. http://malthus.micro.med.umich.edu/lab/usadata/: University of Michigan. Accessed 7 Jan 2015
Knight DA, Ngiow SF, Li M, Parmenter T, Mok S, Cass A et al (2013) Host immunity contributes to the anti-melanoma activity of BRAF inhibitors. J Clin Investig 123(3):1371–1381
Kruger-Krasagakes S, Krasagakis K, Garbe C, Schmitt E, Huls C, Blankenstein T et al (1994) Expression of interleukin 10 in human melanoma. Br J Cancer 70:1182–1185
Labbe K, Danialou G, Gvozdic D, Demoule A, Divangahi M, Boyd JH et al (2010) Inhibition of monocyte chemoattractant protein-1 prevents diaphragmatic inflammation and maintains contractile function during endotoxemia. Critica Care. 14(R187):1–11
Lai X, Friedman A (2017) Combination therapy for melanoma with BRAF/MEK inhibitor and immune checkpoint inhibitor: a mathematical model. BMC Systs Biol 11(1):1–18
Lai X, Stiff A, Duggan M, Wesolowski R, Carson WE III, Friedman A (2018) Modeling combination therapy for breast cancer with BET and immune checkpoint inhibitors. PNAS 115(21):5534–5539
Lavi O, Gottesman MM, Levy D (2012) The dynamics of drug resistance: a mathematical perspective. Drug Resist Updates 15(1–2):90–97
Leonard GD, Fojo T, Bates SE (2003) The role of ABC transporters in clinical practice. Oncologist 8(5):411–424
Liao KL, Bai XF, Friedman A (2014) Mathematical modeling of interleukin-27 induction of anti-tumor T cells response. PLoS ONE 9(3):e91844
Lim S, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ (2016) Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 7(19):28697–28710
Lisiero DN, Soto H, Liau LM, Prins RM (2011) Enhanced sensitivity of IL-2 signaling regulates the clinical responsiveness of IL-12-primed CD8\(^+\) T cells in a melanoma model. J Immunol 186:5068–5077
Luebker SA, Koepsell SA (2019) Diverse mechanisms of BRAF inhibitor resistance in melanoma identified in clinical and preclinical studies. Front Oncol 9(268):1–8
Ma Y, Shurin GV, Peiyuan Z, Shurin MR (2013) Dendritic cells in the cancer microenvironment. J Cancer 4(1):36–44
Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B (2017) The different mechanisms of cancer drug resistance: a brief review. Adv Pharm Bull 7(3):339–348
Manzano JL, Layos L, Bugés C, de los Llanos Gil M, Vila L, Martínez-Cardús EMA (2016) Resistant mechanisms to BRAF inhibitors in melanoma. Ann Transl Med 4(12):237–246
Marino S, Hogue IB, Ray CJ, Kirschner DE (2008) A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theor Biol 254:178–196
Markowitz J, Wang J, Vangundy Z, You J, Yildiz V, Yu L et al (2017) Nitric oxide mediated inhibition of antigen presentation from DCs to CD4+ T cells in cancer and measurement of STAT1 nitration. Sci Rep 7(1):15424–15436
Maute RL, Gordon SR, Mayer AT, McCracken MN, Natarajan A, Ring NG et al (2015) Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging. Proc Natl Acad Sci USA 112(47):E6506–14
Menzies AM, Long GV (2013) Recent advances in melanoma systemic therapy. BRAF inhibitors, CTLA4 antibodies and beyond. Eur J Cancer 49(15):5229–5241
Messerschmidt JL, Prendergast GC, Messerschmidt GL (2016) How cancers escape immune destruction and mechanisms of action for the new significantly active immune therapies: helping nonimmunologists decipher recent advances. Oncologist 21(2):233–243
Muppidi MR, George S (2015) Immune checkpoint inhibitors in renal cell carcinoma. J Targeted Ther Cancer 4:47–52
Oelkrug C, Ramage JM (2014) Enhancement of t cell recruitment and infiltration into tumours. Clin Exp Immunol 178(1):1–8
Ostroumov D, Fekete-Drimusz N, Saborowski M, Kühnel F, Woller N (2018) CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci 75(4):689–713
Ott PA, Henry T, Baranda S, Frleta D, Manches O, Bogunovic D et al (2013) Inhibition of both braf and mek in BRAF(V600E) mutant melanoma restores compromised dendritic cell (DC) function while having differential direct effects on dc properties. Cancer Immunol Immunother 62(4):811–822
Perrot CY, Javelaud D, Mauviel A (2013) Insights into the transforming growth factor-beta signaling pathway in cutaneous melanoma. Ann Dermatol 25(2):135–144
Reddy SM, Reuben A, Wargo JA (2016) Influences of BRAF inhibitors on the immune microenvironment and the rationale for combined molecular and immune targeted therapy. Curr Oncol Rep 18:42
Rockne RC, Hawkins-Daarud A, Swanson KR, Sluka JP, Glazier JA, Macklin P et al (2019) The 2019 mathematical oncology roadmap. Phys Biol 16(4):041005
Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N et al (2014) Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 26(5):623–637
Safarzadeh E, Hashemzadeh S, Duijf PHG, Mansoori B, Khaze V, Mohammadi A et al (2019) Circulating myeloid-derived suppressor cells: an independent prognostic factor in patients with breast cancer. J cell Physiol 234:3515–3525
Sanchez-Laorden B, Viros A, Girotti MR, Pedersen M, Saturno G, Zambon A et al (2014) BRAF inhibitors induce metastasis in RAS mutant or inhibitor-resistant melanoma cells by reactivating MEK and ERK signaling. Sci Signal 7(318):ra30
Shi L, Chen S, Yang L, Li Y (2013) The role of PD-1 and PD-L1 in T cell immune suppression in patients with hematological malignancies. J Hematol Oncol. 6:74
Siewe N, Yakubu AA, Satoskar AR, Friedman A (2017) Granuloma formation in leishmaniasis: a mathematical model. J Theor Biol 412:48–60
Steinberg SM, Shabaneh TB, Zhang P, Martyanov V, Li Z, Malik BT et al (2017) Myeloid cells that impair immunotherapy are restored in melanomas with acquired resistance to BRAF inhibitors. Cancer Res. 77(7):1599–1610
Stiff A, Trikha P, Mundy-Bosse B, McMichael E, Mace TA, Benner B et al (2018) Nitric oxide production by myeloid-derived suppressor cells plays a role in impairing Fc receptor-mediated natural killer cell function. Clin Cancer Res 24(8):1891–1904
Sun X, Hu B (2018) Mathematical modeling and computational prediction of cancer drug resistance. Brief Bioinform 19(6):1382–1399
Tsukumo S, Yasutomo K (2018) Regulation of CD8+ T cells and antitumor immunity by notch signaling. Front Immunol 9(101):1–7
Umansky V, Blattner C, Gebhardt C, Utikal J (2016) The role of myeloid-derived suppressor cells (MDSC) in cancer progression. Vaccines (Basel) 4(36):1–16
Vacaflores A, Freedman SN, Chapman NM, Houtman JCD (2017) Pretreatment of activated human CD8 T cells with IL-12 leads to enhanced TCR-induced signaling and cytokine production. Mol Immunol 81:1–15
Vadlapatla RK, Vadlapudi AD, Pal D, Mitra AK (2013) Mechanisms of drug resistance in cancer chemotherapy: coordinated role and regulation of efflux transporters and metabolizing enzymes. Curr Pharm Des 19(40):7126–7140
Vanichapol T, Chutipongtanate S, Anurathapan U, Hongeng S (2018) Immune escape mechanisms and future prospects for immunotherapy in neuroblastoma. Biomed Res Int 2018:1812535
Vergani E, Di Guardo L, Dugo M, Rigoletto S, Tragni G, Ruggeri R et al (2016) Overcoming melanoma resistance to vemurafenib by targeting CCL2-induced miR-34a, miR-100 and miR-125b. Oncotarget 7(4):4428–4441
Vescovi R, Monti M, Moratto D, Paolini L, Consoli F, Benerini L et al (2018) Collapse of the plasmacytoid dendritic cell compartment in advanced cutaneous melanomas by components of the tumor cell secretome. Cancer Immunol Res 7(1):12–28
Villanueva J, Vultur A, Herlyn M (2011) Resistance to BRAF inhibitors: unraveling mechanisms and future treatment options. Cancer Res 73(23):7137–7140
Wang Y, Zhang X, Yang L, Xue J, Hu G (2018) Blockade of CCL2 enhances immunotherapeutic effect of anti-PD1 in lung cancer. J Bone Oncol 11:27–32
Whiteside TL (2015) The role of regulatory T cells in cancer immunology. Immunotargets Ther 4:159–171
Wooten DJ, Quaranta V (2017) Mathematical model of cell phenotype regulation and reprogramming: make cancer cells sensitive again!. Bioch Biophys Acta (BBA)-Reviews on Cancer 1867(2):167–175
Yang SX, Wei WS, Ouyan QW, Jiang QH, Zou YF, Qu W et al (2016) Interleukin-12 activated CD8+ T cells induces apoptosis in breast cancer cells and reduces tumor growth. Biomed Pharmacother. 84:1466–1471
Young ME (1980) Estimation of diffusion coefficients of proteins. Biotech Bioeng XXII:947–955
Zahreddine H, Borden KLB (2013) Mechanisms and insights into drug resistance in cancer. Front Pharmacol 4(28):1–8
Zanudo JGT, Steinway SN, Albert R (2018) Discrete dynamic network modeling of oncogenic signaling: mechanistic insights for personalized treatment of cancer. Curr Opin Sys Biol 9:1–10
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Friedman, A., Siewe, N. Overcoming Drug Resistance to BRAF Inhibitor. Bull Math Biol 82, 8 (2020). https://doi.org/10.1007/s11538-019-00691-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-019-00691-0