Abstract
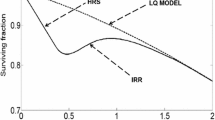
We develop a mathematical model to study the immediate effect of low-dose radiation on the G2 checkpoint and the G2/M transition of the cell cycle via a radiation pathway (the ATM–Chk2 pathway) of an individual mammalian cell. The model consists of a system of nonlinear differential equations describing the dynamics of a network of regulatory proteins that play key roles in the G2/M transition, cell cycle oscillations, and the radiation pathway. We simulate the application of a single pulse of low-dose radiation at different intensities (\(\sim \) 0–0.4 Gy) and times during the latter part of the G2-phase. We use bifurcation analysis to characterize the effect of radiation on the G2/M transition via the ATM–Chk2 pathway. We show that radiation between 0.1 and 0.3 Gy can delay the G2/M transition, and radiation higher than 0.3 Gy can fully activate the G2 checkpoint. Also, our results show that radiation can be low enough to neither delay the G2/M transition nor activate the G2 checkpoint (\(\sim \) 0.1 Gy). Our model supports the idea that the cell response to radiation during G2-phase explains hyper-radiosensitivity and increased radioresistance (HRS/IRR) observed at low dose.
Similar content being viewed by others
References
Bartek J, Falck J, Lukas J (2001) CHK2 kinase—a busy messenger. Nat Rev Mol Cell Biol 2(12):877–86
Bezanson J, Edelman A, Karpinski S, Shah VB (2017) Julia: a fresh approach to numerical computing. SIAM Rev 59(1):65–98
Bodgi L, Foray N (2016) The nucleo-shuttling of the ATM protein as a basis for a novel theory of radiation response: resolution of the linear-quadratic model*. Int J Radiat Biol 92(3):117–131
Bodgi L, Canet A, Pujo-menjouet L, Lesne A (2016) Mathematical models of radiation action on living cells: from the target theory to the modern approaches. A historical and critical review. J Theor Biol 394:93–101
Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ (2001) ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 276(45):42462–42467
Buscemi G, Savio C, Zannini L, Miccichè F, Masnada D, Nakanishi M, Tauchi H, Komatsu K, Mizutani S, Khanna K, Chen P, Concannon P, Chessa L, Delia D (2001) Chk2 activation dependence on Nbs1 after DNA damage. Mol Cell Biol 21(15):5214–5222
Deckbar D, Birraux J, Krempler A, Tchouandong L, Beucher A, Walker S, Stiff T, Jeggo P, Löbrich M (2007) Chromosome breakage after G2 checkpoint release. J Cell Biol 106(6):749–755
Deckbar D, Jeggo PA, Löbrich M (2011) Understanding the limitations of radiation-induced cell cycle checkpoints. Crit Rev Biochem Mol Biol 46(4):271–283
Donzelli M, Draetta GF (2003) Regulating mammalian checkpoints through Cdc25 inactivation. EMBO Rep 4(4):671–677
Enns L, Rasouli-Nia A, Hendzel M, Marples B, Weinfeld M (2015) Association of ATM activation and DNA repair with induced radioresistance after low-dose irradiation. Radiat Prot Dosimetry 166(1–4):131–6
Ermentrout GB, Kopell N (1986) Parabolic bursting in an excitable system coupled with a slow oscillation. SIAM J Appl Math 46(2):233–253
Gérard C, Tyson JJ, Coudreuse D, Novák B (2015) Cell cycle control by a minimal Cdk network. PLOS Comput Biol 11(2):e1004056
Glass L, Mackey MC (1988) From clocks to chaos: the rhythms of life. Princeton University Press, Princeton
Godin M, Delgado FF, Son S, Grover WH, Bryan AK, Tzur A, Jorgensen P, Payer K, Grossman AD, Kirschner MW, Manalis SR (2010) Using buoyant mass to measure the growth of single cells. Nat Methods 7(5):387–390
Goldbeter A, Koshland DE (1981) An amplified sensitivity arising from covalent modification in biological systems. Proc Natl Acad Sci USA 78(11):6840–6844
Goodarzi AA, Noon AT, Deckbar D, Ziv Y, Shiloh Y, Löbrich M, Jeggo PA (2008) ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell 31(2):167–177
Haince JF, Kozlov S, Dawson VL, Dawson TM, Hendzel MJ, Lavin MF, Poirier GG (2007) Ataxia telangiectasia mutated (ATM) signaling network is modulated by a novel poly(ADP-ribose)-dependent pathway in the early response to DNA-damaging agents. J Biol Chem 282(22):16441–16453
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Hartwell L, Weinert T (1989) Checkpoints: controls that ensure the order of cell cycle events. Science 246(4930):629–634
Iliakis G, Wang Y, Guan J, Wang H (2003) DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene 22:5834–5847
Ishikawa A, Yamauchi M, Suzuki K, Yamashita S (2010) Image-based quantitative determination of DNA damage signal reveals a threshold for G2 checkpoint activation in response to ionizing radiation. Genome Integr 1:1–10
Joiner MC, Marples B, Lambin P, Short SC, Turesson I (2001) Low-dose hypersensitivity: current status and possible mechanisms. Int J Radiat Oncol Biol Phys 49(2):379–389
Kozlov SV, Graham ME, Jakob B, Tobias F, Kijas AW, Tanuji M, Chen P, Robinson PJ, Taucher-Scholz G, Suzuki K, So S, Chen D, Lavin MF (2011) Autophosphorylation and ATM activation: additional sites add to the complexity. J Biol Chem 286(11):9107–9119
Löbrich M, Jeggo PA (2007) The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat Rev Cancer 7(11):861–869
Marples B (2004) Is low-dose hyper-radiosensitivity a measure of G2-phase cell radiosensitivity? Cancer Metastasis Rev 23(3–4):197–207
Marples B, Collis SJ (2008) Low-dose hyper-radiosensitivity: past, present, and future. Int J Radiat Oncol Biol Phys 70(5):1310–1318
Marples B, Joiner MC (1993) The response of Chinese hamster V79 cells to low radiation doses: evidence of enhanced sensitivity of the whole cell population. Radiat Res 133(1):41–51
Matsuoka S, Huang M, Elledge SJ (1998) Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science (New York, NY) 282(5395):1893–1897
Medema RH, Macůrek L (2012) Checkpoint control and cancer. Oncogene 31(21):2601–13
Miciak J, Bunz F (2017) Understanding the pluses of pulses. Cell Cycle 16(14):1325
Morgan DO (2007) The cell cycle: principles of control. New Science Press Ltd, London
Murray D, McEwan AJ (2007) Radiobiology of systemic radiation therapy. Cancer Biother Radiopharm 22(1):1–23
Novák B, Tyson JJ (1993) Numerical analysis of a comprehensive model of M-phase control in Xenopus oocyte extracts and intact embryos. J Cell Sci 106:1153–1168
Novák B, Tyson JJ (2008) Design principles of biochemical oscillators. Nat Rev Mol Cell Biol 9(12):981–991
Novák B, Pataki Z, Ciliberto A, Tyson JJ (2001) Mathematical model of the cell division cycle of fission yeast. Chaos 11(1):277–286
Olobatuyi O, de Vries G, Hillen T (2017) A reaction-diffusion model for radiation-induced bystander effects. J Math Biol 75(2):341–372
Olobatuyi O, de Vries G, Hillen T (2018) Effects of G2-checkpoint dynamics on low-dose hyper-radiosensitivity. J Math Biol 77(6–7):1969–1997
Park K, Millet LJ, Kim N, Li H, Jin X, Popescu G, Aluru NR, Hsia KJ, Bashir R (2010) Measurement of adherent cell mass and growth. Proc Natl Acad Sci 107(48):20691–20696
Rackauckas C, Nie Q (2017) DifferentialEquations.jl—a performant and feature-rich ecosystem for solving differential equations in Julia. J Open Res Softw 5(15):15
Rothkamm K, Löbrich M (2003) Evidence for a lack of DNA double-strand break repair in human cells exposed to very low X-ray doses. Proc Natl Acad Sci USA 100(9):5057–62
Scott BR (2010) Multicellular signalling model for DNA double-strand break repair kinetics after low-dose radiation. Int J Low Radiat 7(5):347–358
Short S, Mayes C, Woodcock M, Johns H, Joiner MC (1999) Low dose hypersensitivity in the T98G human glioblastoma cell line. Int J Radiat Biol 75(7):847–55
Taleei R (2018) Modelling Dsb repair kinetics for DNA damage induced by proton and carbon ions. Radiat Prot Dosim 183:75–78
Taleei R, Nikjoo H (2013) The non-homologous end-joining (NHEJ) pathway for the repair of DNA double-strand breaks: I. A mathematical model. Radiat Res 179(5):530–9
Tyson JJ, Novák B (2001) Regulation of the eukaryotic cell cycle: molecular antagonism, hysteresis, and irreversible transitions. J Theor Biol 210(2):249–263
Tyson JJ, Novák B (2015) Models in biology: lessons from modeling regulation of the eukaryotic cell cycle. BMC Biol 13(1):46
Tyson JJ, Csikasz-Nagy A, Novák B (2002) The dynamics of cell cycle regulation. BioEssays 24(12):1095–1109
Tyson JJ, Chen KC, Novák B, Novak B (2003) Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr Opin Cell Biol 15(2):221–231
Vitale I, Galluzzi L, Castedo M, Kroemer G (2011) Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol 12(6):385–392
Zhang P, Wang B, Chen X, Cvetkovic D, Chen L, Lang J, Ma CM (2015) Local tumor control and normal tissue toxicity of pulsed low-dose rate radiotherapy for recurrent lung cancer. Dose Response 13(2):155932581558850
Acknowledgements
We are thankful to Michael Hendzel, David Murray, and John Tyson for their inspiration, discussion, and suggestions. Also, we thank members of the Collaborative Mathematical Biology Group (formerly known as the Centre for Mathematical Biology) at the University of Alberta for their feedback. GC is funded by the Athabasca University Research Fund (Grant Numbers: 22314 and 22725) and GdeV is supported by NSERC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Parameter Values
Appendix: Parameter Values
The Goldbeter–Koshland kinetics model for protein activation is given by
where x is the normalized concentration of the active form of the protein, \(1-x\) is the concentration of the inactive form of the protein, \(v_{1}\) is the maximum activation rate, \(v_{2}\) is the maximum inactivation rate, and \(J_{1}\) and \(J_{2}\) are the Michaelis constants. The smaller the Michaelis constants, the faster the activation/inactivation switch. It can be shown that the activation of x is obtained when
Ultrasensitivity refers to a fast switch in activity when \({v_{1}}\) is close to \({v_{2}}\) and the Michaelis constants are small. Parameter values in the radiation pathway module, such as activation/inactivation rate and Michaelis constants, are carefully chosen so that our simulations match experimental results (see Table 2).
Rights and permissions
About this article
Cite this article
Contreras, C., Carrero, G. & de Vries, G. A Mathematical Model for the Effect of Low-Dose Radiation on the G2/M Transition. Bull Math Biol 81, 3998–4021 (2019). https://doi.org/10.1007/s11538-019-00645-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-019-00645-6