Abstract
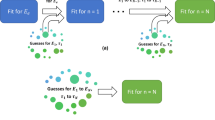
We present a novel framework to parameterise a mathematical model of cell invasion that describes how a population of melanoma cells invades into human skin tissue. Using simple experimental data extracted from complex experimental images, we estimate three model parameters: (i) the melanoma cell proliferation rate, \(\lambda \); (ii) the melanoma cell diffusivity, D; and (iii) \(\delta \), a constant that determines the rate that melanoma cells degrade the skin tissue. The Bayesian sequential learning framework involves a sequence of increasingly sophisticated experimental data from: (i) a spatially uniform cell proliferation assay; (ii) a two-dimensional circular barrier assay; and (iii) a three-dimensional invasion assay. The Bayesian sequential learning approach leads to well-defined parameter estimates. In contrast, taking a naive approach that attempts to estimate all parameters from a single set of images from the same experiment fails to produce meaningful results. Overall, our approach to inference is simple-to-implement, computationally efficient, and well suited for many cell biology phenomena that can be described by low-dimensional continuum models using ordinary differential equations and partial differential equations. We anticipate that this Bayesian sequential learning framework will be relevant in other biological contexts where it is challenging to extract detailed, quantitative biological measurements from experimental images and so we must rely on using relatively simple measurements from complex images.







Similar content being viewed by others
References
Anderson ARA, Quaranta V (2008) Integrative mathematical oncology. Nat Rev Cancer 8:227–34
Beaumont MA, Zhang W, Balding DJ (2002) Approximate Bayesian computation in population genetics. Genetics 162:2025–2035
Bowden LG, Maini PK, Moulton DE, Tang JB, Wang XT, Liu PY, Byrne HM (2014) An ordinary differential equation model for full thickness wounds and the effects of diabetes. J Theor Biol 361:87–100
Browning AP, McCue SW, Simpson MJ (2017) A Bayesian computational approach to explore the optimal duration of a cell proliferation assay. Bull Math Biol 79:1888–1906
Browning AP, McCue SW, Binny RN, Plank MJ, Shah ET, Simpson MJ (2018) Inferring parameters for a lattice-free model of cell migration and proliferation using experimental data. J Theor Biol 437:251–260
Cai AQ, Landman KA, Hughes BD (2007) Multi-scale modeling of a wound-healing cell migration assay. J Theor Biol 245:576–594
Carey TE, Takahashi T, Resnick LA, Oettgen HF, Old LJ (1976) Cell surface antigens of human malignant melanoma: mixed hemadsorption assays for humoral immunity to cultured autologous melanoma cells. PNAS 73:3278–3282
Collis J, Connor AJ, Paczkowski M, Kannan P, Pitt-Francis J, Byrne HM, Hubbard ME (2017) Bayesian calibration, validation and uncertainty quantification for predictive modelling of tumour growth: a tutorial. J R Soc Interface 15:20180318
Daly AD, Gavaghan D, Cooper J, Tavener S (2018) Inference-based assessment of parameter identifiability in nonlinear biological models. Bull Math Biol 79:939–974
Fasano A, Herrero MA, Rodrigo MR (2009) Slow and fast invasion waves in a model of acid-mediated tumour growth. Math Biosci 220:45–56
Fearnhead P, Prangle D (2012) Constructing summary statistics for approximate Bayesian computation: semi-automatic approximate Bayesian computation. J R Stat Soc B 74:419–474
Gatenby RA, Gawlinski ET (1996) A reaction–diffusion model of cancer invasion. Cancer Res 56:5745–5753
Gelman A, Carlin JB, Stern HS, Rubin DB (2004) Bayesian data analysis. CRC Press, Boca Raton
Haridas P, Penington CJ, McGovern JA, McElwain DLS, Simpson MJ (2017a) Quantifying rates of cell migration and cell proliferation in co-culture barrier assays reveals how skin and melanoma cells interact during melanoma spreading and invasion. J Theor Biol 423:13–25
Haridas P, McGovern JA, McElwain DLS, Simpson MJ (2017b) Quantitative comparison of the spreading and invasion of radial growth phase and metastatic melanoma cells in a three-dimensional human skin equivalent model. PeerJ 5:e3754
Haridas P, Browning AP, McGovern JA, McElwain DLS, Simpson MJ (2018) Three-dimensional experiments and individual based simulations show that cell proliferation drives melanoma nest formation in human skin tissue. BMC Syst Biol 12:34
Illowsky B, Dean S (2015) Introductory statistics. OpenStax College, Houston
Jain HV, Richardson A, Meyer-Hermann M, Byrne HM (2014) Exploiting the synergy between Carboplatin and ABT737 in the treatment of ovarian carcinomas. PLoS ONE 9:e81582
Johnston ST, Simpson MJ, McElwain DLS, Binder BJ, Ross JV (2014) Interpreting scratch assays using pair density dynamics and approximate Bayesian computation. Open Biol 4:140097
Landman KA, Pettet GJ (1998) Modelling the action of proteinase and inhibitor in tissue invasion. Math Biosci 154:23–37
Landman KA, Pettet GJ, Newgreen NF (2003) Chemotactic cellular migration: smooth and discontinuous travelling wave solutions. SIAM J Appl Math 63:1666–1681
Landman KA, Simpson MJ, Slater JL, Newgreen DF (2005) Diffusive and chemotactic cellular migration: smooth and discontinuous traveling wave solutions. SIAM J Appl Math 65:1420–1442
Landman KA, Simpson MJ, Pettet GJ (2008) Tactically-driven nonmonotone travelling waves. Physica D 237:678–691
Maclaren OJ, Parker A, Pin C, Carding SR, Watson AJM, Fletcher AG, Byrne HM, Maini PK (2017) A hierarchical Bayesian model for understanding the spatiotemporal dynamics of the intestinal epithelium. PLoS Comput Biol 13:1005688
Maini PK, McElwain DLS, Leavesley DI (2004a) Traveling wave model to interpret a wound-healing cell migration assay for human peritoneal mesothelial cells. Tissue Eng 10:475–482
Maini PK, McElwain DLS, Leavesley D (2004b) Travelling waves in a wound healing assay. Appl Math Lett 17:575–580
Marchant BP, Norbury J, Sherratt JA (2001) Travelling wave solutions to a haptotaxis-dominated model of malignant invasion. Nonlinearity 14:1653–1671
Marchant BP, Norbury J (2002) Discontinuous travelling wave solutions for certain hyperbolic systems. IMA J Appl Math 67:201–224
Marchant BP, Norbury J, Byrne HM (2006) Biphasic behaviour in malignant invasion. Math Med Biol 3:173–196
Massey SC, Assanah MC, Lopez KA, Canoll P, Swanson KR (2012) Glial progenitor cell recruitment drives aggressive glioma growth: mathematical and experimental modelling. J R Soc Interface 9:1757–1766
Mathworks (2018a) Image processing toolbox. https://au.mathworks.com/products/image.html. Accessed Aug 2018
Mathworks (2018b) Gridded and scattered data interpolation, data gridding, piecewise polynomials. https://au.mathworks.com/help/matlab/interpolation.html. Accessed Aug 2018
Mathworks (2018c) Kernel smoothing function estimate for univariate and bivariate data. http://www.mathworks.com/help/stats/ksdensity.html. Accessed Aug 2018
Perumpanani A, Sherratt JA, Norbury J, Byrne HM (1999) A two parameter family of travelling waves with a singular barrier arising from the modelling of extracellular matrix mediated cellular invasion. Physica D 126:145–159
Perumpanani AJ, Marchant BP, Norbury J (2000) Travelling shock waves arising in a model of malignant invasion. SIAM J Appl Math 60:463–476
Sarapata EA, de Pillis LG (2014) A comparison and catalog of intrinsic tumor growth models. Bull Math Biol 76:2010–2024
Sengers BG, Please CP, Oreffo ROC (2007) Experimental characterization and computational modelling of two-dimensional cell spreading for skeletal regeneration. J R Soc Interface 4:1107–1117
Sisson SA, Fan Y, Tanaka MM (2007) Sequential Monte Carlo without likelihoods. PNAS 104:1760–1765
Simpson MJ, Landman KA (2007) Nonmonotone chemotactic invasion: high-resolution simulations, phase plane analysis and new benchmark problems. J Comput Phys 225:6–12
Simpson MJ, McInerney S, Carr EJ, Cuttle L (2017) Quantifying the efficacy of first aid treatments for burn injuries using mathematical modelling and in vivo porcine experiments. Sci Rep 7:10925
Smallbone K, Gavaghan DJ, Gatenby RA, Maini PK (2005) The role of acidity in solid tumour growth and invasion. J Theor Biol 235:476–484
Swanson KR, Rockne RC, Claridge J, Chaplain MA, Alvord EC, Anderson ARA (2011) Quantifying the role of angiogenesis in malignant progression of gliomas: in silico modeling integrates imaging and histology. Cancer Res 71:7366–7375
Toni T, Welch D, Strelkowa N, Ipsen A, Stumpf MPH (2009) Approximate Bayesian computation scheme for parameter inference and model selection in dynamical systems. J R Soc Interface 6:187–202
Treloar KK, Simpson MJ (2013) Sensitivity of edge detection methods for quantifying cell migration assays. PLoS ONE 8:e67389
Treloar KK, Simpson MJ, Haridas P, Manton KJ, Leavesley DI, McElwain DLS, Baker RE (2013) Multiple types of data are required to identify the mechanisms influencing the spatial expansion of melanoma cell colonies. BMC Syst Biol 7:137
Tremel A, Tirtaatmadja N, Hughes BD, Stevens GW, Landman KA, OConnor AJ (2009) Cell migration and proliferation during monolayer formation and wound healing. Chem Eng Sci 64:247–253
Vittadello ST, McCue SW, Gunasingh G, Haass NK, Simpson MJ (2018) Mathematical models for cell migration wit real-time cell cycle dynamics. Biophys J 114:1241–1253
Warne DJ, Baker RE, Simpson MJ (2017) Optimal quantification of contact inhibition in cell populations. Biophys J 113:1920–1924
Warne DJ, Baker RE, Simpson MJ (2018) Multilevel rejection sampling for approximate Bayesian computation. Comput Stat Data Anal 124:71–86
Acknowledgements
This work is supported by the Australian Research Council (DP170100474). We appreciate helpful comments from David Warne, and two anonymous referees.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Browning, A.P., Haridas, P. & Simpson, M.J. A Bayesian Sequential Learning Framework to Parameterise Continuum Models of Melanoma Invasion into Human Skin. Bull Math Biol 81, 676–698 (2019). https://doi.org/10.1007/s11538-018-0532-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-018-0532-1