Abstract
There are a growing number of studies that model immunological processes in the artery wall that lead to the development of atherosclerotic plaques. However, few of these models use parameters that are obtained from experimental data even though data-driven models are vital if mathematical models are to become clinically relevant. We present the development and analysis of a quantitative mathematical model for the coupled inflammatory, lipid and macrophage dynamics in early atherosclerotic plaques. Our modeling approach is similar to the biologists’ experimental approach where the bigger picture of atherosclerosis is put together from many smaller observations and findings from in vitro experiments. We first develop a series of three simpler submodels which are least-squares fitted to various in vitro experimental results from the literature. Subsequently, we use these three submodels to construct a quantitative model of the development of early atherosclerotic plaques. We perform a local sensitivity analysis of the model with respect to its parameters that identifies critical parameters and processes. Further, we present a systematic analysis of the long-term outcome of the model which produces a characterization of the stability of model plaques based on the rates of recruitment of low-density lipoproteins, high-density lipoproteins and macrophages. The analysis of the model suggests that further experimental work quantifying the different fates of macrophages as a function of cholesterol load and the balance between free cholesterol and cholesterol ester inside macrophages may give valuable insight into long-term atherosclerotic plaque outcomes. This model is an important step toward models applicable in a clinical setting.




Similar content being viewed by others
References
Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA (1995) The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol 128(6):1243–1253
Assmann G, Nofer JR (2003) Atheroprotective effects of high-density lipoproteins. Annu Rev Med 54(1):321–341
Brown MS, Goldstein JL (1983) Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem 52(1):223–261
Brown MS, Goldstein JL, Krieger M, Ho Y, Anderson R (1979) Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J Cell Biol 82(3):597–613
Brown MS, Ho Y, Goldstein J (1980) The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J Biol Chem 255(19):9344–9352
Brownell N, Rohatgi A (2016) Modulating cholesterol efflux capacity to improve cardiovascular disease. Curr Opin Lipidol 27(4):398–407
Bulelzai MA, Dubbeldam JL (2012) Long time evolution of atherosclerotic plaques. J Theor Biol 297:1–10
Calvez V, Houot JG, Meunier N, Raoult A, Rusnakova G (2010) Mathematical and numerical modeling of early atherosclerotic lesions. ESAIM Proc Surv 30:14. https://doi.org/10.1051/proc/2010002
Chalmers AD, Cohen A, Bursill CA, Myerscough MR (2015) Bifurcation and dynamics in a mathematical model of early atherosclerosis. J Math Biol 71(6–7):1451–1480
Chen N, Frishman WH (2016) High-density lipoprotein infusion therapy and atherosclerosis: current research and future directions. Cardiol Rev 24(6):298–302
Cobbold C, Sherratt J, Maxwell S (2002) Lipoprotein oxidation and its significance for atherosclerosis: a mathematical approach. Bull Math Biol 64(1):65–95
Crosetto P, Reymond P, Deparis S, Kontaxakis D, Stergiopulos N, Quarteroni A (2011) Fluid-structure interaction simulation of aortic blood flow. Comput Fluids 43(1):46–57
Crouse J, Parks J, Schey H, Kahl F (1985) Studies of low density lipoprotein molecular weight in human beings with coronary artery disease. J Lipid Res 26(5):566–574
Cybulsky MI, Gimbrone MA Jr (1991) Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251(4995):788
De Wilde D, Trachet B, De Meyer G, Segers P (2016) The influence of anesthesia and fluid-structure interaction on simulated shear stress patterns in the carotid bifurcation of mice. J Biomech 49(13):2741–2747
DeVries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I (2005) Cholesterol-induced macrophage apoptosis requires er stress pathways and engagement of the type a scavenger receptor. J Cell Biol 171(1):61–73
El Khatib N, Génieys S, Kazmierczak B, Volpert V (2012) Reaction-diffusion model of atherosclerosis development. J Math Biol 65(2):349–374
Faxon DP, Fuster V, Libby P, Beckman JA, Hiatt WR, Thompson RW, Topper JN, Annex BH, Rundback JH, Fabunmi RP et al (2004) Atherosclerotic vascular disease conference writing group III: pathophysiology. Circulation 109(21):2617–2625
Feintuch A, Ruengsakulrach P, Lin A, Zhang J, Zhou YQ, Bishop J, Davidson L, Courtman D, Foster FS, Steinman DA et al (2007) Hemodynamics in the mouse aortic arch as assessed by MRI, ultrasound, and numerical modeling. Am J Physiol Heart Circ Physiol 292(2):H884–H892
Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA et al (2003) The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol 5(9):781–792
Friedman A, Hao W (2015) A mathematical model of atherosclerosis with reverse cholesterol transport and associated risk factors. Bull Math Biol 77(5):758–781
Frostegard J, Haegerstrand A, Gidlund M, Nilsson J (1991) Biologically modified ldl increases the adhesive properties of endothelial cells. Atherosclerosis 90(2–3):119–126
Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ (1987) Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 316(22):1371–1375
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5(12):953–964
Hansson GK, Libby P (2006) The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 6(7):508–519
Henriksen T, Mahoney EM, Steinberg D (1983) Enhanced macrophage degradation of biologically modified low density lipoprotein. Arterioscler Thromb Vasc Biol 3(2):149–159
Ibragimov A, McNeal C, Ritter L, Walton J (2005) A mathematical model of atherogenesis as an inflammatory response. Mathematical Medicine and Biology 22(4):305–333
Jeng JR, Chang CH, Shih-Ming S, Hui-Chong C (1993) Oxidized low-density lipoprotein enhances monocyte-endothelial cell binding against shear-stress-induced detachment. Biochim Biophys Acta BBA Mol Cell Res 1178(2):221–227
Kontush A, Chapman MJ (2006) Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev 58(3):342–374
Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW (2002) Scavenger receptors class ai/ii and cd36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem 277(51):49982–49988
Leake DS, Rankin SM (1990) The oxidative modification of low-density lipoproteins by macrophages. Biochem J 270(3):741–748
Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y et al (2012) Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med 209(1):123–137
Ley K, Laudanna C, Cybulsky MI, Nourshargh S (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7(9):678–689
Libby P (2002) Inflammation in atherosclerosis. Nature 420:868–874
Libby P, Tabas I, Fredman G, Fisher EA (2014) Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res 114(12):1867–1879
Lin J, Li H, Yang M, Ren J, Huang Z, Han F, Huang J, Ma J, Zhang D, Zhang Z et al (2013) A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep 3(1):200–210
Llodrá J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ (2004) Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Nat Acad Sci USA 101(32):11779–11784
Lodish H, Baltimore D, Berk A, Zipursky SL, Matsudaira P, Darnell J (1995) Molecular cell biology, vol 3. Scientific American Books, New York
Lougheed M, Lum CM, Ling W, Suzuki H, Kodama T, Steinbrecher U (1997) High affinity saturable uptake of oxidized low density lipoprotein by macrophages from mice lacking the scavenger receptor class a type I/II. J Biol Chem 272(20):12938–12944
Mackness M, Abbott C, Arrol S, Durrington P (1993) The role of high-density lipoprotein and lipid-soluble antioxidant vitamins in inhibiting low-density lipoprotein oxidation. Biochem J 294(3):829–834
Melmed R, Karanian P, Berlin R (1981) Control of cell volume in the J774 macrophage by microtubule disassembly and cyclic AMP. J Cell Biol 90(3):761–768
Milo R (2013) What is the total number of protein molecules per cell volume? A call to rethink some published values. Bioessays 35(12):1050–1055
Moireau P, Xiao N, Astorino M, Figueroa CA, Chapelle D, Taylor CA, Gerbeau JF (2012) External tissue support and fluid-structure simulation in blood flows. Biomech Model Mechanobiol 11(1–2):1–18
Moore KJ, Tabas I (2011) Macrophages in the pathogenesis of atherosclerosis. Cell 145(3):341–355
Moore KJ, Sheedy FJ, Fisher EA (2013) Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 13(10):709–721
Morel D, DiCorleto PE, Chisolm G (1984) Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arterioscler Thromb Vasc Biol 4(4):357–364
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8(12):958–969
Nagano Y, Arai H, Kita T (1991) High density lipoprotein loses its effect to stimulate efflux of cholesterol from foam cells after oxidative modification. Proc Nat Acad Sci 88(15):6457–6461
National Institutes of Health, and National Heart, Lung, and Blood Institute et al (2001) ATP III guidelines at-a-glance quick desk reference. NIH publication (01-3305)
Ougrinovskaia A, Thompson RS, Myerscough MR (2010) An ode model of early stages of atherosclerosis: mechanisms of the inflammatory response. Bull Math Biol 72(6):1534–61. https://doi.org/10.1007/s11538-010-9509-4
Parton A, McGilligan V, O’Kane M, Baldrick FR, Watterson S (2016) Computational modelling of atherosclerosis. Brief Bioinform 17(4):562–575
Peiffer V, Sherwin SJ, Weinberg PD (2013) Does low and oscillatory wall shear stress correlate spatially with early atherosclerosis? A systematic review. Cardiovasc Res 99(2):242–250
Phillips MC, Gillotte KL, Haynes MP, Johnson WJ, Lund-Katz S, Rothblat GH (1998) Mechanisms of high density lipoprotein-mediated efflux of cholesterol from cell plasma membranes. Atherosclerosis 137:S13–S17
Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ (2011) Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of apoe-/-mice during disease regression. J Clin Investig 121(5):2025–2036
Prosi M, Zunino P, Perktold K, Quarteroni A (2005) Mathematical and numerical models for transfer of low-density lipoproteins through the arterial walls: a new methodology for the model set up with applications to the study of disturbed lumenal flow. J Biomech 38(4):903–917
Rajman I, Eacho PI, Chowienczyk P, Ritter J (1999) Ldl particle size: an important drug target? Br J Clin Pharmacol 48(2):125–133
Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D et al (2013) Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 19(9):1166–1172
Ross R (1999) Atherosclerosis—an inflammatory disease. N Engl J Med 340(2):115–126
Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W (2005) Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol 25(6):1256–1261
Soran H, Schofield JD, Liu Y, Durrington PN (2015) How HDL protects LDL against atherogenic modification: paraoxonase 1 and other dramatis personae. Curr Opin Lipidol 26(4):247–256
Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW (1994) A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 89(5):2462–2478
Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW (1995) A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. Circulation 92(5):1355–1374
Steingberg D (1989) Beyond cholesterol modification of low density lipoprotein that increase its atherogenesity. N Engl J Med 320:915–924
Stender S, Zilversmit D (1981) Transfer of plasma lipoprotein components and of plasma proteins into aortas of cholesterol-fed rabbits. Molecular size as a determinant of plasma lipoprotein influx. Arterioscler Thromb Vasc Biol 1(1):38–49
Stocker R, Keaney JF (2004) Role of oxidative modifications in atherosclerosis. Physiol Rev 84(4):1381–1478
Tabas I (2005) Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol 25(11):2255–2264
Tabas I (2017) 2016 Russell ross memorial lecture in vascular biology. Arterioscler Thromb Vasc Biol 37(2):183–189
Tabas I, Marathe S, Keesler GA, Beatini N, Shiratori Y (1996) Evidence that the initial up-regulation of phosphatidylcholine biosynthesis in free cholesterol-loaded macrophages is an adaptive response that prevents cholesterol-induced cellular necrosis proposed role of an eventual failure of this response in foam cell necrosis in advanced atherosclerosis. J Biol Chem 271(37):22773–22781
Teerlink T, Scheffer PG, Bakker SJ, Heine RJ (2004) Combined data from LDL composition and size measurement are compatible with a discoid particle shape. J Lipid Res 45(5):954–966
Thon MP, Hemmler A, Glinzer A, Mayr M, Wildgruber M, Zernecke-Madsen A, Gee MW (2017) A multiphysics approach for modeling early atherosclerosis, biomechanics and modeling in mechanobiology. Biomech Model Mechanobiol. https://doi.org/10.1007/s10237-017-0982-7
Tompkins RG (1991) Quantitative analysis of blood vessel permeability of squirrel monkeys. Am J Physiol Heart Circ Physiol 260(4):H1194–H1204
Véniant MM, Withycombe S, Young SG (2001) Lipoprotein size and atherosclerosis susceptibility in apoe-/- and ldlr-/- mice. Arterioscler Thromb Vasc Biol 21(10):1567–1570
Virmani R, Burke AP, Kolodgie FD, Farb A (2002) Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol 15(6):439–446
Wang W, Lee Y, Lee CH (2013) Review: the physiological and computational approaches for atherosclerosis treatment. Int J Cardiol 167(5):1664–1676
Williams KJ, Tabas I (1995) The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 15(5):551–561
Witztum JL, Steinberg D (1991) Role of oxidized low density lipoprotein in atherogenesis. J Clin Investig 88(6):1785
Yang N, Vafai K (2006) Modeling of low-density lipoprotein (LDL) transport in the artery—effects of hypertension. Int J Heat Mass Transf 49(5):850–867
Yao PM, Tabas I (2000) Free cholesterol loading of macrophages induces apoptosis involving the fas pathway. J Biol Chem 275(31):23807–23813
Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS (2008) Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem 283(34):22930–22941
Zi Z (2011) Sensitivity analysis approaches applied to systems biology models. IET Syst Biol 5(6):336–346
Zohdi T, Holzapfel G, Berger S (2004) A phenomenological model for atherosclerotic plaque growth and rupture. J Theor Biol 227(3):437–443
Acknowledgements
We thank Dr. Christina Bursill of the South Australian Health and Medical Research Institute for helpful discussions. This work was supported by the German Research Foundation (DFG) and the Technical University of Munich (TUM) in the framework of the Open Access Publishing Program. Michael W. Gee and Moritz P. Thon acknowledge the financial support given by the International Graduate School of Science and Engineering of the TUM under the project BioMat01, A Multiscale Model of Atherosclerosis. Mary R. Myerscough and Hugh Z. Ford acknowledge support from an Australian Research Council Discovery Project Grant (to Mary R. Myerscough).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that no conflicts of interest exist
Appendices
Appendix 1: Experiment-Specific Parameters of Submodels
The following Tables 5, 6 and 7 contain the experiment-specific parameters of the mathematical submodels of in vitro systems in analogy to the experimental setups in Henriksen et al. (1983), Leake and Rankin (1990), Mackness et al. (1993), Brown et al. (1979) and Brown et al. (1980).
Appendix 2: Least-Squares Fits of Submodels
The following Figs. 5, 6, 7, 8, 9 and 10 contain all least-squares fits of the mathematical submodels of in vitro systems to experimental results in Henriksen et al. (1983), Leake and Rankin (1990), Mackness et al. (1993), Brown et al. (1979), Brown et al. (1980) and Yao and Tabas (2000). Appendix 1: Experiment-specific parameters of submodels and Table 4 give the experiment-specific and least-squares fitted parameters of the submodels, respectively.
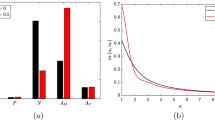
Comparison of results of the mathematical submodel of LDL modification and ingestion (submodel 1) with results from various experimental setups in Henriksen et al. (1983). Least-squares fits of the simulated ingestion of native and modified LDL per macrophage \(\frac{a_{\mathrm {Ing}}(T_\mathrm {Ing})}{m_\mathrm {Ing}}\) to experimental results a in Henriksen et al. (1983), Fig. 1 for varying ingestion time periods \(T_\mathrm {Ing}\), b in Henriksen et al. (1983), Fig. 2 for varying modification time periods \(T_\mathrm {Mod}\) and c in Henriksen et al. (1983), Fig. 5 for varying initial LDL ingestion concentrations \({\ell }_{\mathrm {Ing},0}\) (Color figure online)
Comparison of results of the mathematical submodel of LDL modification and ingestion (submodel 1) with results from various experimental setups in Leake and Rankin (1990). Least-squares fits of the simulated ingestion of native and modified LDL per macrophage \(\frac{a_{\mathrm {Ing}}(T_\mathrm {Ing})}{m_\mathrm {Ing}}\) to experimental results (a in Leake and Rankin (1990), Fig. 1a for varying modification time periods \(T_\mathrm {Mod}\) and b in Leake and Rankin (1990), Fig. 4 for varying initial LDL ingestion concentrations \({\ell }_{\mathrm {Ing},0}\) (Color figure online)
Comparison of results of the mathematical submodel of the HDL protection against LDL modification (submodel 2) with results from various experimental setups in Mackness et al. (1993). Least-squares fits of the simulated lipid peroxide content per lipoprotein \(\frac{N_{\tilde{\ell }}{\tilde{\ell }}(T_\mathrm {Mod})}{{\ell }_{0}}\) and the HDL protection to experimental results a in Mackness et al. (1993), Fig. 4 for varying modification time periods \(T_\mathrm {Mod}\) and b in Mackness et al. (1993), Fig. 5 for varying initial HDL concentrations \(h_0\) (Color figure online)
Comparison of results of the mathematical submodel of cholesterol cycle and reverse cholesterol transport (submodel 3) with results from various experimental setups in Brown et al. (1979). Least-squares fits of the simulated concentration of intracellular free cholesterol \(\frac{f(T_\text {Cho})}{m}\) to the experimental results a in Brown et al. (1979), Fig. 1a and b in Brown et al. (1979), Fig. 1b for varying experimental time periods \(T_\text {Cho}\) (Color figure online)
Comparison of results of the mathematical submodel of cholesterol cycle and reverse cholesterol transport (submodel 3) with results from various experimental setups in Brown et al. (1980). Least-squares fits of the simulated concentration of intracellular free cholesterol \(\frac{f(T_\text {Cho})}{m}\), intracellular cholesterol ester \(\frac{b(T_\text {Cho})}{m}\) and excreted cholesterol \(\frac{r(T_\text {Cho})}{m}\) per macrophage to the experimental results a in Brown et al. (1980), Fig. 1a for varying experimental time periods \(T_\text {Cho}\), b in Brown et al. (1980), Fig. 1b for varying experimental time periods \(T_\text {Cho}\), c in Brown et al. (1980), Fig. 1c for varying experimental time periods \(T_\text {Cho}\), d in Brown et al. (1980), Fig. 2a for varying additions of HDL \(h_0\), e in Brown et al. (1980), Fig. 4 for varying additions of HDL \(h_0\) and f in Brown et al. (1980), Fig. 7b for varying experimental time periods \(T_\text {Cho}\) (Color figure online)

Comparison of least-squares fit of simulated macrophage apoptosis to experimental results in Yao and Tabas (2000), Fig. 4a (Color figure online)
Appendix 3: Proof of Proposition 1
In the following we prove Proposition 1. To prove the positivity of \({\ell }\), i.e., \({\ell }(t)\ge 0 \; \forall t\ge 0\) it is sufficient to note that \({\ell }(0)=0\) and that \({\ell }(t)=0\) implies
due to the strict positivity of the parameters. The positivity of \(h, m\) and \({\tilde{\ell }}\) can be proved in an analogue fashion. Since \(\frac{f(0)}{m(0)}=f_0 \ge f_\mathrm {Min}\) and \(\frac{f(t)}{m(t)}=f_\mathrm {Min}\) implies
it holds \(\frac{f(t)}{m(t)} \ge f_\mathrm {Min} \; \forall t \ge 0\). This also implies the positivity of \(f\). Given that \(\frac{f(0)}{m(0)}=f_0 < f_\mathrm {Max}\) and that \(\frac{f(t)}{m(t)}\rightarrow f_\mathrm {Max}\) implies
it follows \(\frac{f(t)}{m(t)} \le f_\mathrm {Max} \; \forall t \ge 0\). Hence, we conclude that
The positivity of \(b\) follows since \(b(0)=0\) and \(b(t)=0\) implies
which finishes the proof of 1. (It also follows that the time-dependent solution \(({\ell }(t),{\tilde{\ell }}(t),h(t),f(t),b(t),m(t)), t\ge 0\) of the initial value problem is unique and smooth because the smoothness of the right-hand side of ordinary differential equation (6) is now straight-forward to show.)
Using (14)
holds, and by solving this ordinary differential inequality with associated initial condition \(m(0)=m_0\) it follows
In an analogue way, the upper bound for \(m(t)\) can be found, leading to
which finishes the proof of 2.
The boundedness of \({\ell }(t)\) is given by
since the solution of the ordinary differential inequality (with associated initial condition \({\ell }(0)=0\)) is bounded by
In an analogue way, the boundedness of \(h(t)\) is proved. We show boundedness of \({\tilde{\ell }}(t)\) under the condition \(\frac{r_{\ell }}{H}< \mu _{\tilde{\ell }}m_\mathrm {Min}\) by a proof by contradiction. Hence, let \({\tilde{\ell }}(t)\) be unbounded, i.e., \({\tilde{\ell }}(t) \rightarrow \infty \) as \(t\rightarrow \infty \) and \(\frac{r_{\ell }}{H}< \mu _{\tilde{\ell }}m_\mathrm {Min}\). It follows
which is in contradiction to the assumed unboundedness of \({\tilde{\ell }}(t)\). Hence, this finishes the proof of Proposition 1. \(\square \)
Rights and permissions
About this article
Cite this article
Thon, M.P., Ford, H.Z., Gee, M.W. et al. A Quantitative Model of Early Atherosclerotic Plaques Parameterized Using In Vitro Experiments. Bull Math Biol 80, 175–214 (2018). https://doi.org/10.1007/s11538-017-0367-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-017-0367-1