Abstract
Molecular motors such as kinesin and dynein are responsible for transporting material along microtubule networks in cells. In many contexts, motor dynamics can be modelled by a system of reaction–advection–diffusion partial differential equations (PDEs). Recently, quasi-steady-state (QSS) methods have been applied to models with linear reactions to approximate the behaviour of the full PDE system. Here, we extend this QSS reduction methodology to certain nonlinear reaction models. The QSS method relies on the assumption that the nonlinear binding and unbinding interactions of the cellular motors occur on a faster timescale than the spatial diffusion and advection processes. The full system dynamics are shown to be well approximated by the dynamics on the slow manifold. The slow manifold is parametrized by a single scalar quantity that satisfies a scalar nonlinear PDE, called the QSS PDE. We apply the QSS method to several specific nonlinear models for the binding and unbinding of molecular motors, and we use the resulting approximations to draw conclusions regarding the parameter dependence of the spatial distribution of motors for these models.
















Similar content being viewed by others
Change history
04 January 2018
We apologize for the error in the references.
References
Baas PW, Deitch JS, Black MM, Banker GA (1988) Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci 85(21):8335–8339
Bhat D, Gopalakrishnan M (2012) Effectiveness of a dynein team in a tug of war helped by reduced load sensitivity of detachment: evidence from the study of bidirectional endosome transport in D. discoideum. Phys Biol 9(4):46003
Blasius TL, Reed N, Slepchenko BM, Verhey KJ (2013) Recycling of kinesin-1 motors by diffusion after transport. PLoS One 8(9):e76081
Bressloff P, Newby J (2013) Stochastic models of intracellular transport. Rev Mod Phys 85(1):135–196
Bressloff PC, Newby JM (2011) Quasi-steady-state analysis of two-dimensional random intermittent search processes. Phys Rev E 83(6):061139
Burton PR (1988) Dendrites of mitral cell neurons contain microtubules of opposite polarity. Brain Res 473(1):107–115
Chowdhury D, Schadschneider A, Nishinari K (2005) Traffic phenomena in biology: from molecular motors to organisms. Traffic Granul Flow 2(4):223–238
Ciandrini L, Romano MC, Parmeggiani A (2014) Stepping and crowding of molecular motors: statistical kinetics from an exclusion process perspective. Biophys J 107(5):1176–1184
Dauvergne D, Edelstein-Keshet L (2015) Application of quasi-steady state methods to molecular motor transport on microtubules in fungal hyphae. J Theor Biol 379:47–58
Dixit R, Ross JL, Goldman YE, Holzbaur ELF (2008) Differential regulation of dynein. Science 319(February):8–11
Fink G, Steinberg G (2006) Dynein-dependent motility of microtubules and nucleation sites supports polarization of the tubulin array in the fungus Ustilago maydis. Mol Biol Cell 17(7):3242–3253
Gou J, Edelstein-Keshet L, Allard J (2014) Mathematical model with spatially uniform regulation explains long-range bidirectional transport of early endosomes in fungal hyphae. Mol Biol Cell 25(16):2408–2415
Hendricks AG, Perlson E, Ross JL, Schroeder HW, Tokito M, Holzbaur ELF (2010) Motor coordination via a tug-of-war mechanism drives bidirectional vesicle transport. Curr Biol 20(8):697–702
Klumpp S, Lipowsky R (2005) Cooperative cargo transport by several molecular motors. Proc Natl Acad Sci USA 102(48):17284–17289
Leduc C, Padberg-Gehle K, Varga V, Helbing D, Diez S, Howard J (2012) Molecular crowding creates traffic jams of kinesin motors on microtubules. Proc Natl Acad Sci USA 109(16):6100–6105
Mallik R, Rai AK, Barak P, Rai A, Kunwar A (2013) Teamwork in microtubule motors. Trends Cell Biol 23(11):575–582
Mattila PK, Lappalainen P (2008) Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol 9(6):446–454
McVicker DP, Chrin LR, Berger CL (2011) The nucleotide-binding state of microtubules modulates kinesin processivity and the ability of Tau to inhibit kinesin-mediated transport. J Biol Chem 286(50):42873–42880
Müller MJI, Klumpp S, Lipowsky R (2008) Motility states of molecular motors engaged in a stochastic tug-of-war. J Stat Phys 133(6):1059–1081
Nambiar R, McConnell RE, Tyska MJ (2010) Myosin motor function: the ins and outs of actin-based membrane protrusions. Cell Mol Life Sci 67(8):1239–1254
Newby J, Bressloff PC (2010a) Local synaptic signaling enhances the stochastic transport of motor-driven cargo in neurons. Phys Biol 7(3):036004
Newby JM, Bressloff PC (2010b) Quasi-steady state reduction of molecular motor-based models of directed intermittent search. Bull Math Biol 72(7):1840–1866
Parmeggiani A, Franosch T, Frey E (2004) Totally asymmetric simple exclusion process with langmuir kinetics. Phys Rev E 70(4):046101
Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ (2006) Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol 16(21):2166–2172
Reichenbach T, Frey E, Franosch T (2007) Traffic jams induced by rare switching events in two-lane transport. New J Phys 9(6):159
Rzadzinska AK, Schneider ME, Davies C, Riordan GP, Kachar B (2004) Stereocilia functional architecture and self-renewal. J Cell Biol 164(6):887–897
Schneider ME, Dose AC, Salles FT, Chang W, Erickson FL, Burnside B, Kachar B (2006) A new compartment at stereocilia tips defined by spatial and temporal patterns of myosin IIIa expression. J Neurosci 26(40):10243–10252
Schuster M, Kilaru S, Fink G, Collemare J, Roger Y, Steinberg G (2011a) Kinesin-3 and dynein cooperate in long-range retrograde endosome motility along a nonuniform microtubule array. Mol Biol Cell 22(19):3645–3657
Schuster M, Lipowsky R, Assmann M-A, Lenz P, Steinberg G (2011b) Transient binding of dynein controls bidirectional long-range motility of early endosomes. Proc Natl Acad Sci USA 108(9):3618–3623
Schwander M, Kachar B, Müller U (2010) Review series: the cell biology of hearing. J. Cell Biol 190(1):9–20
Shubeita GT (2012) Intracellular transport: relating single-molecule properties to in vivo function. Compr Biophys 4:287–297
Smith DA, Simmons RM (2001) Models of motor-assisted transport of intracellular particles. Biophys J 80(1):45–68
Steinberg G (2011) Motors in fungal morphogenesis: cooperation versus competition. Curr Opin Microbiol 14(6):660–667
Steinberg G, Wedlich-Soldner R, Brill M, Schulz I (2001) Microtubules in the fungal pathogen Ustilago maydis are highly dynamic and determine cell polarity. J Cell Sci 114(Pt 3):609–622
Stone MC, Roegiers F, Rolls MM (2008) Microtubules have opposite orientation in axons and dendrites of drosophila neurons. Mol Biol Cell 19(10):4122–4129
Yochelis A, Gov NS (2016) Reaction–diffusion–advection approach to propagating aggregates of molecular motors. Phys D 318–319:1–15
Yochelis A, Ebrahim S, Millis B, Cui R, Kachar B, Naoz M, Gov NS (2015) Self-organization of waves and pulse trains by molecular motors in cellular protrusions. Sci Rep 5:13521
Acknowledgements
M. J. W. was supported by the NSERC Discovery Grant 81541. L. E. K. was supported by an NSERC Discovery Grant 41870. C. Z. was supported by the NSERC Discovery Grant to L. E. K. and T. S. was supported by a USRA position funded by an NSERC Discovery Grant to L. E. K.
Author information
Authors and Affiliations
Corresponding author
Additional information
Michael J. Ward and Leah Edelstein-Keshet are co-supervising authors.
A correction to this article is available online at https://doi.org/10.1007/s11538-017-0386-y.
Appendices
Appendix A: Microtubule Density and Binding by Motor Complexes
1.1 Kinesin Model with Non-uniform MT Density
To explicitly incorporate the possibility that MT density, m(x) (as well as fraction of MT pointing to the right, P(x)) varies across the cell, we can write the kinesin model equations as
This modification of the model introduces another factor into coefficients that are already spatially dependent, but otherwise leaves the model structure unchanged. Hence, the techniques in the paper apply as before with \(k_{\text {bm}}m(x) \) replacing the parameter \(k_{\text {b}}\).
For the purposes of our proof-of-concept analysis, we now restrict attention to uniform MT density so that \(m(x)\equiv m_0\) is a constant. Then the model for kinesin is given by (75) as below, with the assignment
That is, the binding constant \(k_{\text {b}}\) is understood to represent the net rate of binding, which includes both the per-MT-binding rate and the MT density.
1.2 Kinesin–Dynein Model and the Function Q(x)
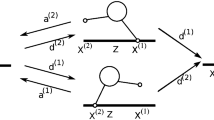
The kinesin–dynein model simplifies the binding of free motor complexes into states that move right with probability Q(x) and left with probability \(1-Q(x)\). We consider the case of motor complexes that all have \(n_k\) kinesin and \(n_d\) dynein components. (The case of complexes with a variety of motor numbers can be handled by considering the mean composition of a complex or the mean ratio between the two motor types.) Let us also define the parameters \(k_{bd}\) and \(k_{bk}\) as the binding rates for a (single) dynein and for a (single) kinesin to a MT, and consider m(x) as the local MT density. Then we can decompose the quantity \(k_{\text {b}}Q\) in the model as follows:
This related the aggregate binding rate to the probability that a kinesin binds to right-pointing MT and that dynein binds to left-pointing MT. Similarly,
Since such details merely substitute one spatially dependent function for another, the analysis we have described carries over as before.
Appendix B: Scaling the Models and the QSS Reduction
1.1 The Kinesin Model
We consider the kinesin model with uniform MT density and demonstrate its scaling here. This system is
We define T by
Then T is the total amount of motors inside the cell, and \({\rho =T/L_0}\) is the average density of motors in the cell.
Scale space, time, and densities as follows:
where \(y^{\star }={p^{\text {R}}}^{\star }+{p^{\text {L}}}^{\star }+{p^{\text {U}}}^{\star } \) is the total scaled density. We have scaled distance by the cell length and time by the time that a motor takes to walk across the cell. The densities of each state are scaled by the average motor density across the cell.
Then we can recast the total amount as
Taking out the constant factor of \(\rho L_0\equiv T\) from the integral results in
which leads to
With this scaling, the integral of the total scaled density is unity, which we assume throughout our numerical computations.
Substituting the scaled variables into the PDE system (75) leads to
Then we can consider two cases, depending on whether the function g is linear or not.
Case I: g is linear In this case, we can eliminate the factor \(\rho \) from every term. Dividing each term in the equations by \(v \rho /L_0\) and dropping the stars leads to
where D, \(\varepsilon \), and \(k_{\text {a}}\) are defined by
In this case, these dimensionless parameters represent, respectively, the ratio of (time to be transported:time to diffuse) across the cell (D), the ratio of (time spent unbound:time to walk) across the cell (\(\varepsilon \)), and the ratio of (time spent unbound:time spent bound) (\(k_{\text {a}}\)).
Case II: g is Michaelian or Hill
Then, (76) becomes
Define a new constant \(A\equiv {K/\rho }\). This constant is the ratio of the motor concentration at which the binding rate is half-maximal to the average motor density in the cell. Divide numerator and denominator of the Hill function by \(\rho ^n\). Further, divide every term in the equations by \(v \rho /L_0\) as before. Then we obtain after rearranging and dropping the starred notation is
where D and \(\varepsilon \) are as before, but \(k_{\text {a}}\) now depends on whether g is a Michaelis–Menten or a Hill function. This holds for any Hill coefficient n. Note that, in particular, for the case \(n=1\), which is the Michaelian case considered, we have that
where \(c\equiv {1/A}={\rho /K}\). In (80) and (81) \(k_{\text {a}}\) is defined by
In either case, the parameter \(k_{\text {a}}\) describes the ratio of time spent bound to the time spent unbound, mediated by the nonlinear binding kinetics.
Finally, we scale the boundary conditions in (5) to get
together with
1.2 Kinesin–Dynein Model Scaling
Define \(k_{\text {c}}\equiv k_{\text {rl}}- k_{\text {lr}}\). Then the model can be written as
Scale all variables as before. Then terms of the form \((k_{\text {c}}/k_{\text {u}}) p^{\text {R}}p^{\text {L}}\) will lead to the form \((k_{\text {c}}/k_{\text {u}}) \rho {p^{\text {R}}}^{\star } \rho {p^{\text {L}}}^{\star }\), so that what remains, after cancelling out a factor of \(v_{\text {r}}\rho /L_0 \) from every term in each equation, and dropping the starred quantities, is
where the parameters are
Here \(\rho \) is the average density of motors inside the cell. These dimensionless parameters represent, respectively, the (left:right) walking speed ratio (v), the ratio of (time to be transported:time to diffuse) across the cell (D), the ratio of (time spent unbound:time to walk) across the cell (\(\varepsilon \)), the ratio of (time spent unbound:time spent bound) (\(k_{\text {a}}\)), and the turning parameter k, which represents the ratio of (net right–left direction switches:unbinding rate). We comment that the average density of motors \(\rho \) enters into the turning rate parameter due to the nonlinearity of the model with respect to the turning of motors when they collide on a MT.
1.2.1 Details of QSS Reduction of Kinesin–Dynein Model
Next, we provide some details of the QSS reduction of the kinesin–dynein model. Upon setting \(f_2=f_3=0\) in (49) we get the two equations
It is convenient to let \(p^{\text {L}}\) be the free variable and parameterize the quasi-steady-state in terms of \(p^{\text {L}}=\alpha \). By solving (88) for \(p^{\text {R}}\) and \(p^{\text {U}}\), we get the quasi-steady-state solution \(\mathbf {p}^0\) as given in (51). We then readily show that the nonzero eigenvalues \(\lambda _{\pm }\) of the Jacobian of the kinetics satisfy the quadratic equation given in (52) and (53). A necessary and sufficient condition for \(\hbox {Re}(\lambda _{\pm })<0\) is that \(\sigma _1<0\) and \(\sigma _2>0\) in (53). To establish this result we need some properties of H(Q) defined in (53). We first observe that \(H(0)=1\), so that trivially \(\sigma _1<0\) and \(\sigma _2>0\) when \(Q=0\). Then, since \(H^{\prime }(Q)=-{(1+k\alpha )/(1+k\alpha -Q)^2}<0\), it follows that \(\sigma _1<0\) and \(\sigma _2>0\) on \(0\le Q\le 1\) provided that we can show that \(\sigma _1<0\) and \(\sigma _2>0\) when \(Q=1\). These inequalities do hold at \(Q=1\), since by using \(H(1)={(k\alpha -1)/(k\alpha )}\) we readily obtain that \(\sigma _1=-1-k_{\text {a}}-k\alpha \) and \(\sigma _2=k\alpha (1+k_{\text {a}})>0\) when \(Q=1\). This proves that \(\hbox {Re}(\lambda _{\pm })<0\) for any Q in \(0\le Q\le 1\). As a result, \(\mathbf {p}^0\) defined in (51) is a slow manifold in the sense of Definition (3.1) for any Q in \(0\le Q\le 1\). Finally, by using \(\mathbf {p}^0\) and the operator M, as defined in (49), in the solvability condition (24), we readily derive the QSS PDE model (54).
1.3 Myosin Model Scaling
We carry out similar scaling for the myosin model characterized by
When we scale variables just as before, the terms \(\left( {p^{\text {B}}}\right) ^2 p^{\text {W}}\) will lead to the forms \(\left( \rho {{p^{\text {B}}}}^{\star }\right) ^2 (\rho {p^{\text {W}}}^{\star })\). This will result in a constant factor \(\rho ^2\) that remains after cancelling out \(\rho \) from all terms in the equation. As a result, we will obtain, upon dropping the starred quantities,
where the dimensionless parameters v, D, \(\varepsilon \), \(k_{\text {bw}}\), and \(k_{\text {b}}\) are defined by
Recall that \(\rho \) is the average density of motors inside the cell. These dimensionless parameters represent, respectively, the bound:walking motor speed ratio (v), the ratio of (time to be transported:time to diffuse) across the cell (D), the ratio of (time spent unbound:time to walk) across the cell (\(\varepsilon \)), the interaction parameter \(k_{\text {bw}}\), which represents the ratio of (net rate of collisions that result in direction change:unbinding rate), and the ratio of (time spent unbound:time spent bound) (\(k_{\text {b}}\)). Note that the average density of motors \(\rho \) enters into the interaction rate parameter due to the nonlinearity of the model with motor–motor interaction.
1.4 Non-Spatial Myosin Model
In Sect. 4.3, we seek to determine whether the Type I or Type II QSS PDE better approximates the behaviour of the full myosin system. To understand the behaviour, we study the non-spatial myosin model kinetics through a phase-plane analysis, where the advection and diffusive processes in (90) are neglected.
The non-spatial myosin model kinetics are described by the following system of ODEs:
where time has been scaled to remove the \(\varepsilon \) dependence. Due to conservation of mass, we can write \(p^{\text {U}}= 1 - p^{\text {W}}- p^{\text {B}}\). This facilitates the reduction of this system of three equations to a system of two equations:
With \(k_{\text {bw}}= 25\) and \(k_{\text {b}}= 3\), a phase-plane analysis (see Fig. 17) reveals the existence of an unstable manifold which divides the \((p^{\text {W}},p^{\text {B}})\) plane into two regions. For initial conditions below this unstable manifold, the system converges to a steady-state with \(p^{\text {B}}= 0\), but \(p^{\text {W}}> 0\), as in Type I QSS. For initial conditions above this unstable manifold, the system converges to a steady-state with \(p^{\text {B}}> 0\), as in Type II QSS.
Phase-plane analysis of the non-spatial myosin model. A phase-plane analysis of the non-spatial myosin model (93) reveals the existence of an unstable manifold that divides \((p^{\text {W}},p^{\text {B}})\) space into two regions. For initial conditions below the unstable manifold, the system tends to a steady-state with \(p^{\text {B}}=0\), but for initial conditions above the unstable manifold, the system tends to a steady-state with \(p^{\text {B}}> 0\) (Color figure online)
Appendix C: Numerics for the Steady-State of the QSS PDEs
In this appendix we show how to numerically compute the steady-state solution of the QSS PDEs by recasting the non-local problem into an initial boundary value problem (IBVP), which is amenable to a numerical shooting method.
For the QSS PDE associated with the kinesin model (29) of Sect. 4.1, the steady-state problem is
where \(g(\alpha )\) is either the saturated binding model (34) or the Hill function (48). To reformulate (94), we define N(x) by
Then, (94) is equivalent to the ODE system
with \(N(0)=-1\). We then specify \(\alpha (0)=\beta \), where \(\beta \) is a value to be determined. We solve the IBVPs (96) for various values of \(\beta \) and output the quantity \(N(1;\beta )\). In this numerical shooting procedure, Newton’s method on \(\beta \) is then used to satisfy the required terminal constraint \(N(1;\beta )=0\).
A similar approach can be used to compute steady-state solutions of the QSS PDE (54) for the kinesin–dynein model of Sect. 4.2 subject to the total mass constraint \(\int _{0}^{1} y(x)\, \mathrm{d}x=1\). In place of (96) we obtain
with \(N(0)=-1\) and \(\alpha (0)=\beta \), where \(\beta >0\) is a shooting parameter determined numerically by satisfying the terminal constraint \(N(1;\beta )=0\).
Finally, we consider steady-state solutions of the QSS PDE (68) for the myosin model of Sect. 4.3 subject to the total mass constraint \(\int _{0}^{1} y(x)\, \mathrm{d}x=1\). In place of (96) we get
with \(N(0)=-1\) and \(\alpha (0)=\beta \), where \(\beta >0\) is computed numerically to satisfy the constraint \(N(1;\beta )=0\). A steady-state solution exists only when \(k_{\text {bw}}\alpha ^2>1\) on \(0\le x\le 1\).
To numerically determine the boundary in parameter space where \(k_{\text {bw}}\alpha ^2>1\) holds on \(0\le x\le 1\) for the steady-state when \(0<v<1\), it is convenient to reformulate (98). We define \(A(x)\equiv \sqrt{k_{\text {bw}}} \alpha (x)\) to transform (98) to
A steady-state solution to the QSS PDE exists only when \(A(x)>1\) on \(0\le x\le 1\). Since (99) implies that A(x) is monotonic in x whenever \(A>1\), then it is possible that \(A\rightarrow 1^+\) only for \(x\rightarrow 0^+\) or \(x\rightarrow 1^-\). However, since \(A\rightarrow {1/\sqrt{v}}>1\) on the infinite line as \(x\rightarrow \infty \), it follows that we can only have \(A\rightarrow 1^+\) as \(x\rightarrow 0^+\). To determine the local behaviour as \(A\rightarrow 1^+\) and \(x\rightarrow 0^+\), we calculate from (99) that \({\mathrm{d}A/\mathrm{d}x}\sim c_1{(1-v)/[2(A-1)]}\) and \({\mathrm{d}N/\mathrm{d}x}\sim 2c_2\). This yields the local behaviour
For a fixed v and \(D>0\), with \(0<v<1\), to determine the region in the parameter space \(k_{\text {bw}}\) versus \(k_{\text {b}}\) where \(A(x)>1\) on \(0\le x\le 1\), we proceed as follows. We fix \(c_1\) in (99), numerically integrate the IBVPs (99) with the local behaviour (100) imposed at some \(x=\delta \), with \(0<\delta \ll 1\), and numerically shoot on the value of \(c_2\) for which \(N(1;c_2)=0\). From (99), this determines \(k_{\text {b}}\) and \(k_{\text {bw}}\) as \(k_{\text {b}}=c_1 D\) and \(k_{\text {bw}}= \left[ {(k_{\text {b}}+1)/(k_{\text {b}}c_2)}\right] ^2\).
Appendix D: Boundary Layer Analysis
In this appendix we determine the appropriate boundary conditions for our QSS PDEs, and we analyse the boundary layers near \(x=0,1\). We focus our discussion on general three-component systems on \(0\le x\le 1\) of the form
where \(v_1\), \(v_2\), D are positive \({\mathscr {O}}(1)\) constants, \(\varepsilon \ll 1\), and the kinetics \(f_j=f_j(p_1,p_2,p_3)\) for \(j=1,\ldots ,3\), satisfy the conservation condition
By imposing the mass constraint \(\partial _t \int _{0}^{1} \left( p_1+p_2+p_3\right) \, dx =0\), and setting \(p_1(0,t)=p_2(1,t)=0\), we obtain the following boundary conditions for (101a):
We assume that there is a unique one-parameter family \(\mathbf {p}^0(\alpha )\equiv (p_1^0(\alpha ),p_2^0(\alpha ),p_3^0(\alpha ))^T\) of solutions to the leading-order problem \(\mathbf {f}=(f_1,f_2,f_3)^T=\mathbf {0}\), and that \(\mathbf {p}^0\) is a slow manifold for (101) in the sense of Definition 3.1. This is the leading-order outer solution, valid away from boundary layers at \(x=0,1\). Then, as shown in Sect. 3, \(\alpha =\alpha (x,t)\) satisfies the QSS PDE (24a), which can be written as
We now determine an appropriate boundary condition for (102) as \(x\rightarrow 0^+\) by analysing the boundary layer structure for (101) near the left endpoint \(x=0\). As \(x\rightarrow 0^+\), we obtain from the outer solution that
where we have defined \(p_{j0}^0\equiv p_j^{0}(\alpha (0,t))\) for \(j=1,\ldots ,3\).
We will only analyse in detail the region near \(x=0\), as a similar analysis can be done near \(x=1\). For \(t={\mathscr {O}}(1)\) the two possible dominant balances for the spatial derivatives in (101a) near \(x=0\) are \(x={\mathscr {O}}(\sqrt{\varepsilon })\) and \(x={\mathscr {O}}(\varepsilon )\). On the wider such scale, we let \(\xi ={x/\sqrt{\varepsilon }}\) to obtain from (101a) that
To leading-order we obtain that \(f_1=f_2=0\), so that from (101b) we must have \(f_3=0\). As a result, we obtain to leading-order that \(p_1\sim p_{10}^0\), \(p_2\sim p_{20}^0\), and \(p_3\sim p_{30}^0\). This implies that our QSS approximation is still valid when \(x={\mathscr {O}}(\sqrt{\varepsilon })\).
Next, we analyse the region where \(x={\mathscr {O}}(\varepsilon )\). Upon introducing \(\eta \equiv {x/\varepsilon }\), we obtain from (101a) that
From (101c), the boundary conditions for this system are
while the asymptotic matching conditions, as obtained from (103), are that
For \(t={\mathscr {O}}(1)\), we neglect the asymptotically negligible left-hand sides of (105a) to obtain
By adding the equations in (106), and using the conservation condition (101b), we obtain upon integration in \(\eta \) that, for all \(\eta >0\),
where A is independent of \(\eta \). By evaluating this expression at \(\eta =0\), (105b) yields that \(A=0\). With \(A=0\), we then evaluate (107) as \(\eta \rightarrow \infty \) by using the matching condition (105c). This yields
This key result shows that to obtain the boundary condition at \(x=0\) for the QSS PDE for \(\alpha (x,t)\) we can simply substitute the outer approximation \(p_1=p_1^0(\alpha )\), \(p_2=p_2^0(\alpha )\), and \(p_3=p_3^0(\alpha )\), into the first condition of (101c). In this sense, the QSS PDE inherits the no-flux boundary condition (101c) at \(x=0\). We remark that a similar analysis can be done near \(x=1\), with the analogous result that
To complete the boundary layer analysis near \(x=0\), we expand
and obtain from the first two equations in (106), together with (107) with \(A=0\), the following boundary layer problem on \(0<\eta <\infty \):
Although the first two equations for \(p_1\) and \(p_2\) are uncoupled from \({\mathscr {P}}_3\), in general it is not possible to calculate \(p_1\) and \(p_2\) analytically, especially when \(f_1\) and \(f_2\) are nonlinear in \(p_1\) and \(p_2\). However, the system for \(p_1\) and \(p_2\) are readily studied in the phase-plane.
We remark that a similar boundary layer analysis can be done near \(x=1\). To study this boundary layer, we now define \(\eta ={(1-x)/\varepsilon }\). We readily find in place of (110a) and (110b) that
Here \(p_{j1}^{0}\equiv p_j^{0}(\alpha (1,t))\), for \(j=1,\ldots ,3\).
1.1 The Kinesin Model
For the kinesin model (25) of Sect. 4.1, the boundary layer system (110) can be solved explicitly. With the QSS approximation \(\mathbf {p}^0\), as given in (27), we identify \(v_1=v_2=1\), \(p_1=p^{\text {R}}\), \(p_2=p^{\text {L}}\), and \(p_3=p^{\text {U}}\). From (27), we calculate that \(p_{10}^0=k_{\text {a}}P(0) g(\alpha _0)\), \(p_{20}^0=k_{\text {a}}\left[ 1-P(0)\right] g(\alpha _0)\), and \(p_{30}^0=\alpha (0)\), where \(\alpha _0\equiv \alpha (0,t)\). Therefore, using the reaction kinetics in (25), (110) becomes
The solution with \(p_1(0)=0\) and \(p_2\rightarrow p_{20}^0\) as \(\eta \rightarrow \infty \), is simply \(p_1=p_{10}^{0}(1-e^{-\eta })\), and \(p_2=p_{20}^{0}\). Then, \({\mathscr {P}}_3\) is obtained up to a constant by integrating the last equation in (112). In this way, we obtain the boundary layer solution for \(x={\mathscr {O}}(\varepsilon )\) that
where the constant \(A_3\) can only be determined from a two-term outer QSS solution, which is intractable analytically. This analysis shows two key features. Firstly, the right-moving motors have a classic boundary layer behaviour when \(x={\mathscr {O}}(\varepsilon )\). Secondly, for \(x={\mathscr {O}}(\varepsilon )\) the unbound kinesin motor density \(p^{\text {U}}\) differs from its outer approximation only by an error \({\mathscr {O}}({\varepsilon /D})\). A similar calculation can be done for the boundary layer near \(x=1\) using (111). We leave the details to the reader.
1.2 The Kinesin–Dynein Model
For the kinesin–dynein model (49), the boundary layer equations (110) for the layer near \(x=0\) is analysed via the phase-plane. Using \(\mathbf {f}\) in (49), and setting \(v_1=1\) and \(v_2=v\), (110a) and (110b) on \(0<\eta <\infty \) become
where \(p_{30}^0={(k\alpha _0+1)\alpha _0/[k_a(k\alpha _0+1-Q)]}\). To analyse (114) in the phase-plane, it is convenient to introduce new variables \(q_1(\eta )\) and \(q_2(\eta )\) defined by
In terms of \(q_1\) and \(q_2\), (114) transforms to the two-component dynamical system
As a function of \(r_1\), we have \(r_2=0\) when \(r_1=0\), \(r_2\rightarrow Q<1\) as \(r_1\rightarrow \infty \), and that \(r_2\) is monotone increasing in \(r_1\) since \({dr_2/dr_1}={[Q(1-Q)]/(r_1+1-Q)^2}>0\) holds for \(0<Q<1\). It follows that \(0<r_2<1\) for any \(r_1>0\).
Qualitative analysis of boundary layer behaviour of the kinesin–dynein model. Phase portraits of \(q_2\) versus \(q_1\) for boundary layer solutions of the kinesin–dynein model near \(x=0\) (a) and near \(x=1\) (b) from (116) and (117), respectively. In a there is a unique value \(q_2=q_2^{0}\) at \(q_1=0\) for which (116) has a solution with \((q_1,q_2)\rightarrow (1,1)\) as \(\eta \rightarrow +\infty \). In b there is a unique value \(q_1=q_1^{0}\) at \(q_2=0\) for which (117) has a solution with \((q_1,q_2)\rightarrow (1,1)\) as \(\eta \rightarrow +\infty \). The parameter values of \(r_1\), \(r_2\), and v for b are those consistent with Fig. 7. a \(r_1 = 2.0\), \(r_2 = 0.5\), \(v = 0.5\). b \(r_1 = 1.69\), \(r_2 = 0.85\), \(v = 0.5\) (Color figure online)
By calculating the Jacobian \(J_g\) of \(g_1\) and \(g_2\) at the equilibrium state \(q_1=q_2=1\), we find that
so that \(q_1=q_2=1\) is a saddle point for the dynamics. In Fig. 18a we plot the phase portrait \(q_2\) versus \(q_1\) and nullclines for (116) for representative values \(r_1=2\), \(r_2=0.5\), and \(v=0.5\). We observe that the \(q_2\) nullcline intersects the \(q_2\) axis at \(q_2=1-r_2\in (0,1)\) since \(0<r_2<1\). This plot indicates the existence of a unique value \(q_2(0)=q_2^{0}>1-r_2\) for which (116) has a solution with \((q_1,q_2)\rightarrow (1,1)\) as \(\eta \rightarrow +\infty \). This qualitative analysis confirms the existence of a boundary layer solution near \(x=0\) for the kinesin–dynein model for all range of parameters.
A similar phase-plane analysis can be performed to analyse the boundary layer system (111) near \(x=1\). In place of (116), we obtain that
where in place of (115), \(r_1\) and \(r_2\) are now defined by \(r_1 = k \alpha _1\) and \(r_2\equiv {Qr_1/(r_1 + 1-Q)}\), where \(\alpha _1=\alpha \) at \(x=1\). In Fig. 18b we plot the phase portrait and nullclines for (117) for \(r_1=1.69\), \(r_2=0.85\), and \(v=0.5\), which corresponds to the parameter values used in the caption of Fig. 7. This phase portrait shows the existence of a unique value \(q_1(0)=q_1^{0}\) for which (117) has a solution with \((q_1,q_2)\rightarrow (1,1)\) as \(\eta \rightarrow +\infty \). Our computations yield \(q_1^{0}\approx 1.95\), so that from (115) we get \(p_1\approx 0.83\) at \(x=1\).
1.3 The Myosin Model
For the full myosin transport model (90), the boundary layer equations (110a)–(110b) near \(x=0\) can be studied qualitatively in the phase-plane. Upon setting \(v_1=1\) and \(v_2=v\), (110a) and (110b) on \(0<\eta <\infty \) become
where \(p_{30}^0={\left( \alpha _0+{1/[k_{\text {bw}}\alpha _0]}\right) /k_{\text {b}}}\) and \(\alpha _0=\alpha (0,t)\). We conveniently introduce new variables \(q_1\) and \(q_2\) defined by
so that in terms of \(r\equiv k_{\text {bw}}\alpha _0^2\), (118) becomes
At the equilibrium state \(q_1=q_2=1\), the determinant of the Jacobian \(J_g\) of \(g_1\) and \(g_2\) is \(\hbox {det}(J_g) = {(1-r)/v}\). Therefore, \(\hbox {det}(J_g) <0\) and \(q_1=q_2=1\) is a saddle point if \(r\equiv k_{\text {bw}}\alpha _0^2>1\). In Fig. 19a we plot the phase portrait of \(q_2\) versus \(q_1\) and nullclines for (120) for the representative values \(r=5\) and \(v=0.5\). We observe that there is a unique value \(q_2(0)=q_2^{0}\) for which (120) has a solution with \((q_1,q_2)\rightarrow (1,1)\) as \(\eta \rightarrow +\infty \). As such, there is always a boundary layer solution near \(x=0\) for the myosin model.
Qualitative analysis of boundary layer behaviour of the myosin model. Phase portraits of \(q_2\) versus \(q_1\) for boundary layer solutions of the myosin model near \(x=0\) (a) and near \(x=1\) (b) from (120) and (121), respectively. In a there is a unique value \(q_2=q_2^{0}\) at \(q_1=0\) for which (120) has a solution with \((q_1,q_2)\rightarrow (1,1)\) as \(\eta \rightarrow +\infty \). However, for the right boundary layer, the phase-plane in b there is no value \(q_1=q_1^{0}>0\) at \(q_2=0\) for which \((q_1,q_2)\rightarrow (1,1)\) as \(\eta \rightarrow \infty \). a \(r = 5\), \(v = 0.5\). b \(r = 5\), \(v = 0.5\) (Color figure online)
A similar boundary layer system near \(x=1\) can be obtained from (111) for the myosin model. In place of (120), we obtain that
where r is now defined by \(r=k_{\text {bw}}\alpha _1^2\) with \(\alpha _1=\alpha (1,t)\). Although the equilibrium point \(q_1=q_2=1\) is a saddle point of (121) whenever \(r>1\), the phase portrait in the \(q_2\) versus \(q_1\) plane shown in Fig. 19b shows that there is no value \(q_1(0)=q_1^{0}>0\) on \(q_2=0\) for which \((q_1,q_2)\rightarrow (1,1)\) as \(\eta \rightarrow \infty \).
As such, we conclude for the Type II QSS approximation (64) for the myosin model that there is no steady-state boundary layer solution near \(x=1\) that allows the extra boundary condition \(p^{\text {B}}=0\) at \(x=1\) to be satisfied.
Rights and permissions
About this article
Cite this article
Zmurchok, C., Small, T., Ward, M.J. et al. Application of Quasi-Steady-State Methods to Nonlinear Models of Intracellular Transport by Molecular Motors. Bull Math Biol 79, 1923–1978 (2017). https://doi.org/10.1007/s11538-017-0314-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-017-0314-1