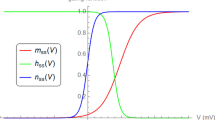
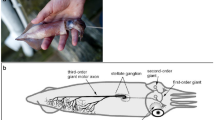
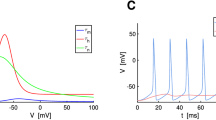
Abstract
The Hodgkin and Huxley equations have served as the benchmark model in electrophysiology since 1950s. But it suffers from four major drawbacks. Firstly, it is only phenomenological not mechanistic. Secondly, it fails to exhibit the all-or-nothing firing mechanism for action potential generation. Thirdly, it lacks a theory for ion channel opening and closing activation across the cell membrane. Fourthly, it does not count for the phenomenon of voltage-gating which is vitally important for action potential generation. In this paper, a mathematical model for excitable membranes is constructed by introducing circuit characteristics for ion pump exchange, ion channel activation, and voltage-gating. It is demonstrated that the model is capable of re-establishing the Nernst resting potentials, explicitly exhibiting the all-or-nothing firing mechanism, and most important of all, filling the long-lasting theoretical gap by a unified theory on ion channel activation and voltage-gating. It is also demonstrated that the new model has one half fewer parameters but fits significantly better to experiment than the HH model does. The new model can be considered as an alternative template for neurons and excitable membranes when one looks for simpler models for mathematical studies and for forming large networks with fewer parameters.










Similar content being viewed by others
References
Agin D (1963) Some comments on the Hodgkin–Huxley equations. J Theor Biol 5(2):161–170
Agin D (1972) Excitability phenomena in membranes. Found Math Biol 1:253–277
Almers W (1978) Gating currents and charge movements in excitable membranes. Rev Physiol Biochem Pharmacol 82:96–190
Armstrong CM, Bezanilla F (1973) Currents related to movement of the gating particles of the sodium channels. Nature 242:459–461
Arvanitaki A, Chalazonitis N (1949) Prototypes dinteractions neuroniques et transmissions synaptiques-donnees bioelectriques de preparations cellulaires. Archives des sciences physiologiques 3(4):547–565
Benoit E, Callot J L, Diener F, Diener (1981) Chasse au canard (première partie). Collect Math 32(1):37–76
Bernstein J (1912) Electrobiologie braunschweig. Friedr. Vieweg und Sohn, Braunschweig, Germany, p 87
Carpenter D, Gunn R (1970) The dependence of pacemaker discharge of Aplysia neurons upon Na\(^+\) and Ca\(^{++}\). J Cell Physiol 75(1):121–127
Chay TR, Keizer J (1983) Minimal model for membrane oscillations in the pancreatic beta-cell. Biophys J 42(2):181
Cole KS (1932) Surface forces of the Arbacia egg. J Cell Comp Physiol 1(1):1–9
Cole KS (1940) Permeability and impermeability of cell membranes for ions. Cold Spring Harb Symp Quant Biol 8:110–122
Cole KS (1949) Dynamic electrical characteristics of the squid axon membrane. Archives des sciences physiologiques 3(2):253–258
Cole KS (1968) Membranes, ions, and impulses: a chapter of classical biophysics, vol 5. University of California Press, Oakland
Connor J, Stevens C (1971) Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol 213(1):31–53
Deng B (2004) Food chain chaos with canard explosion. Chaos Interdiscip J Nonlinear Sci 14(4):1083–1092
Deng B (2009) Conceptual circuit models of neurons. J Integr Neurosci 8(03):255–297
Deng B (2014) An inverse problem: trappers drove hares to eat lynx. Preprint
Deng B, Estes A, Grieb B, Richard D, Hinds B, Hebets E (2014) A male spider’s ornamentation polymorphism maintained by opposing selection with two niches. J Theor Biol 357:103–111
FitzHugh R (1961) Impulses and physiological states in theoretical models of nerve membrane. Biophys J 1(6):445
Frazier WT, Kandel ER, Kupfermann I, Waziri R, Coggeshall RE (1967) Morphological and functional properties of identified neurons in the abdominal ganglion of Aplysia californica. J Neurophysiol 30(6):1288–1351
Goldman DE (1943) Potential, impedance, and rectification in membranes. J Gen Physiol 27(1):37–60
Hindmarsh J, Rose R (1984) A model of neuronal bursting using three coupled first order differential equations. Proc R Soc Lond Ser B Biol Sci 221(1222):87–102
Hodgkin AL, Katz B (1949) The effect of sodium ions on the electrical activity of the giant axon of the squid. J Physiol 108(1):37–77
Hodgkin AL, Huxley AF (1952a) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117(4):500–544
Hodgkin AL, Huxley AF (1952b) Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol 116(4):449–472
Hodgkin A, Huxley A (1952c) The components of membrane conductance in the giant axon of Loligo. J Physiol 116(4):473–496
Hodgkin AL, Huxley AF (1952d) The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol 116(4):497–506
Hodgkin AL, Huxley A, Katz B (1952) Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol 116(4):424–448
Li C-L, Jasper H (1953) Microelectrode studies of the electrical activity of the cerebral cortex in the cat. J Physiol 121(1):117–140
Moore JW (1959) Excitation of the squid axon membrane in isosmotic potassium chloride. Nature 183:265–266
Morris C, Lecar H (1981) Voltage oscillations in the barnacle giant muscle fiber. Biophys J 35(1):193
Partridge LD, Stevens C (1976) A mechanism for spike frequency adaptation. J Physiol 256(2):315–332
Yamamoto K (1965) Negative resistance and electro-kinetic cross-phenomenon in ionic solution. J Phys Soc Jpn 20:1727
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, B. Alternative Models to Hodgkin–Huxley Equations. Bull Math Biol 79, 1390–1411 (2017). https://doi.org/10.1007/s11538-017-0289-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-017-0289-y