Abstract
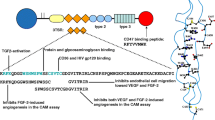
Tumor growth and progression are critically dependent on the establishment of a vascular support system. This is often accomplished via the expression of pro-angiogenic growth factors, including members of the vascular endothelial growth factor (VEGF) family of ligands. VEGF ligands are overexpressed in a wide variety of solid tumors and therefore have inspired optimism that inhibition of the different axes of the VEGF pathway—alone or in combination—would represent powerful anti-angiogenic therapies for most cancer types. When considering treatments that target VEGF and its receptors, it is difficult to tease out the differential anti-angiogenic and anti-tumor effects of all combinations experimentally because tumor cells and vascular endothelial cells are engaged in a dynamic cross-talk that impacts key aspects of tumorigenesis, independent of angiogenesis. Here we develop a mathematical model that connects intracellular signaling responsible for both endothelial and tumor cell proliferation and death to population-level cancer growth and angiogenesis. We use this model to investigate the effect of bidirectional communication between endothelial cells and tumor cells on treatments targeting VEGF and its receptors both in vitro and in vivo. Our results underscore the fact that in vitro therapeutic outcomes do not always translate to the in vivo situation. For example, our model predicts that certain therapeutic combinations result in antagonism in vivo that is not observed in vitro. Mathematical modeling in this direction can shed light on the mechanisms behind experimental observations that manipulating VEGF and its receptors is successful in some cases but disappointing in others.








Similar content being viewed by others
References
Botelho F, Pina F, Lunet N (2010) VEGF and prostatic cancer: a systematic review. Eur J Cancer Prev 19(5):385–392
Byrne HM (2010) Dissecting cancer through mathematics: from the cell to the animal model. Nat Rev Cancer 10(3):221–230
Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C (2011) Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 15(6):1239–1253
Ciric C, Ciffroy P, Charles S (2012) Use of sensitivity analysis to identify influential and non-influential parameters within an aquatic ecosystem model. Ecol Model 246:119–130
Cunningham SA, Tran TM, Arrate MP, Brock TA (1999) Characterization of vascular endothelial cell growth factor interactions with the kinase insert domain-containing receptor tyrosine kinase: a real time kinetic study. J Biol Chem 274(26):18421–18427
Del Monte U (2009) Does the cell number 10(9) still really fit one gram of tumor tissue? Cell Cycle 8(3):505–506
Ellis LM, Hicklin DJ (2008) VEGF-targeted therapy: mechanisms of anti-tumor activity. Nat Rev Cancer 8:579–591
Ferrara N (2002) VEGF and the quest for tumor angiogenesis factors. Nat Rev Cancer 2:795–803
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9(6):669–676
Fujita K, Sano D, Kimura M, Yamashita Y, Kawakami M, Ishiguro Y et al (2007) Anti-tumor effects of bevacizumab in combination with paclitaxel on head and neck squamous cell carcinoma. Oncol Rep 18(1):47–51
Gasparini G (2000) Prognostic value of vascular endothelial growth factor in breast cancer. Oncologist 5(Suppl 1):37–44
Goel S, Wong AH-K, Jain RK (2012) Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb Perspect Med 2(3):a006486
Gotink KJ1, Verheul HM (2010) Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis 13(1):1–14
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M et al (2002) VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA 99:11393–11398
Honey K (2009) Good and bad news for an antiangiogenic therapy. J Clin Investig 119(6):1400
Jain HV, Nor JE, Jackson TL (2008) Modeling the VEGF-Bcl-2-CXCL8 pathway in intratumoral angiogenesis. Bull Math Biol 70(1):89–117
Jain HV, Nor JE, Jackson TL (2009) Quantification of endothelial cell-targeted anti-Bcl-2 therapy and its suppression of tumor growth and vascularization. Mol Cancer Ther 8(1):2926–2936
Jubb AM, Pham TQ, Hanby AM, Frantz GD, Peale FV et al (2004) Expression of vascular endothelial growth factor, hypoxia inducible factor 1\(\alpha \), and carbonic anhydrase IX in human tumours. J Clin Pathol 57:504–512
Kaneko T, Zhang Z, Mantellini MG, Karl E, Zeitlin B, Verhaegen M et al (2007) Bcl-2 orchestrates a cross-talk between endothelial and tumor cells that promotes tumor growth. Cancer Res 67(20):9685–9693
Kendrew J, Eberlein C, Hedberg B, Smith NR et al (2011) An antibody targeted to VEGFR-2 Ig domains 4-7 inhibits VEGFR-2 activation and VEGFR-2-dependent angiogenesis without affecting ligand binding. Mol Cancer Ther 10(5):770–783
Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS et al (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis sup-presses tumour growth in vivo. Nature 362:841–844
King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F et al (2004) Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 67(2):139–151
Kohn-Luque, de Back W, Yamaguchi Y, Yoshimura K, Herrero MA, Miura T (2013) Dynamics of VEGF matrix-retention in vascular network patterning. Phys Biol 10(6):066007
Krupitskaya Y, Wakelee HA (2009) Ramucirumab, a fully human mAb to the transmembrane signaling tyrosine kinase VEGFR-2 for the potential treatment of cancer. Curr Opin Investig Drugs 10:597–605
Lin YS, Nguyen C, Mendoza JL, Escandon E, Fei D, Meng YG et al (1999) Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factor. J Pharmacol Exp Ther 288(1):371–378
Lin MI, Sessa WC (2004) Antiangiogenic therapy: creating a unique “window” of opportunity. Cancer Cell 6(6):529–531
Loges S, Schmidt T, Carmeliet P (2010) Mechanisms of resistance to anti-angiogenic therapy and development of third-generation anti-angiogenic drug candidates. Genes Cancer 1(1):12–25
Lu D, Shen J, Vil MD, Zhang H, Jimenez X, Bohlen P et al (2003) Tailoring in vitro selection for a picomolar affinity human antibody directed against vascular endothelial growth factor receptor 2 for enhanced neutraliz- ing activity. J Biol Chem 278:43496–43507
Mac Gabhann F, Yang MT, Popel AS (2005) Monte Carlo simulations of VEGF binding to cell surface receptors in vitro. Biochim Biophys Acta 1746(2):95–107
Mac Gabhann F, Popel AS (2007) Dimerization of VEGF receptors and implications for signal transduction: a computational study. Biophys Chem 128:125–139
Mac Gabhann F, Popel AS (2007) Interactions of VEGF isoforms with VEGFR-1, VEGFR-2, and neuropilin in vivo: a computational model of human skeletal muscle. Am J Physiol Heart Circ Physiol 292(1):H459–74
McMahon G (2000) VEGF receptor signaling in tumor angiogenesis. Oncologist 5(Suppl 1):3–10
Morris MD (1991) Factorial sampling plans for preliminary computational experiments. Technometrics 33:161–174
Moserle L, Jimenez-Valerio G, Casanovas O (2014) Antiangiogenic therapies: going beyond their limits. Cancer Discov 4(1):31–41
Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z (1999) Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 13(1):9–22
Nor JE, Christensen J, Mooney DJ, Polverini PJ (1999) Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol 154(2):375–384
Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E et al (2012) Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15(2):171–185
Peirce SM (2008) Computational and mathematical modeling of angiogenesis. Microcirculation 15(8):739–751
Perfahl H, Byrne HM, Chen T, Estrella V, Alarcon T, Lapin A et al (2011) Multiscale modelling of vascular tumour growth in 3D: the roles of domain size and boundary conditions. PLoS One 6(4):e14790
Pianosi F, Sarrazin F, Wagener T (2015) A matlab toolbox for global sensitivity analysis. Environ Model Softw 70:80–85
Reed JC, Tsujimoto Y, Epstein SF, Cuddy M, Slabiak T, Nowell PC et al (1989) Regulation of bcl-2 gene expression in lymphoid cell lines containing normal #18 or t(14;18) chromosomes. Oncogene Res 4(4):271–282
Shweiki D, Neeman M, Itin A, Keshet E (1995) Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: Implications for tumor angiogenesis. Proc Natl Acad Sci USA 92(3):768–772
Siemann DW (2011) The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor vascular disrupting agents. Cancer Treat Rev 37:63–74
Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S et al (2010) Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 28(5):780–787
Szabo E, Schneider H, Seystahl K, Rushing EJ, Herting F, Weidner KM, Weller M (2016) Autocrine VEGFR1 and VEGFR2 signaling promotes survival in human glioblastoma models in vitro and in vivo. Neuro Oncol 18(9):1242–1252
Tong M, Lloyd B, Pei P, Mallery SR (2008) Human head and neck squamous cell carcinoma cells are both targets and effectors for the angiogenic cytokine. VEGF J Cell Biochem 105(5):1202–1210
Van Tubergen E, Vander Broek R, Lee J, Wolf G, Carey T, Bradford C et al (2011) Tristetraprolin regulates interleukin-6, which is correlated with tumor progression in patients with head and neck squamous cell carcinoma. Cancer 117(12):2677–2689
von Tiedemann B, Bilitewski U (2002) Characterization of the vascular endothelial growth factor-receptor interaction and determination of the recombinant protein by an optical receptor sensor. Biosens Bioelectron 17(11–12):983–991
Wang D, Lehman RE, Donner DB, Matli MR, Warren RS, Welton ML (2002) Expression and endocytosis of VEGF and its receptors in human colonic vascular endothelial cells. Am J Physiol Gastrointest Liver Physiol 282(6):G1088–1096
Wang Z, Butner JD, Kerketta R, Cristini V, Deisboeck TS (2015) Simulating cancer growth with multiscale agent-based modeling. Semin Cancer Biol 30:70–78
Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J et al (2000) PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res 60:2178–2189
Wu Y, Hooper AT, Zhong Z, Witte L, Bohlen P, Rafii S, Hicklin DJ (2006) The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int J Cancer 1:119(7):1519–1529
Yao J, Wu X, Zhuang G et al (2011) Expression of a functional VEGFR-1 in tumor cells is a major determinant of anti-PlGF antibodies efficacy. PNAS 108(28):11590–11595
Yen P, Finley SD, Engel-Stefanini MO, Popel AS (2011) A two-compartment model of VEGF distribution in the mouse. PLoS One 6(11):e27514
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
1.1 In Vitro Treatment Equations
The complete set of model equations used for simulating in vitro, pre-treatment tumor growth is described in the main text. For the targeted therapies we are considering, the tumor cell, endothelial cell and Bcl-2 equations do not change. Below we describe the modifications to the model that arise due to treatment.
VEGF-Binding Equations: The equations below describe VEGF binding, and they are identical to those presented in the main text, except for the addition of the last two terms in Eqs. (20)–(23), which represent the binding dynamics of anti-VEGF, VEGFR1 or VEGFR2 antibodies.
VEGF, VEGFR1, VEGFR2 Therapy Equations: The binding dynamics for an anti-VEGF antibody (\(X_V\)), an anti-VEGFR1 antibody (\(X_{R1}\)) and an anti-VEGFR2 antibody (\(X_{R2}\)) are described below:
1.2 In Vivo Treatment Equations
The model equations used for simulating tumor cells, endothelial cells and drug pharmacokinetics for anti-VEGF therapy in vivo are described in detail in the main text. Below we present the remaining model equations associated with treatment for the in vivo situation.
Intratumoral VEGF: Intratumoral VEGF (A) is governed by Eq. (34). The first eight terms of the equation, representing binding, decay and production, are described in detail in the main text. The last terms in the VEGF equation represent the response to therapies that target the VEGF.
VEGFR1 and VEGFR2: The equations representing VEGF receptor dynamics on ECs and TCs remain also largely unchanged from the in vitro case described in the main text. The only addition is the therapeutic binding of VEGFR1 and VEGFR2 antibodies, where appropriate. The first three terms in each of the equations below represent the response to VEGF of each variable (\(R_i\) for \(i = 1, 2 \equiv \) free VEGFR1/VEGFR2 receptors on ECs and/or TCs, and \(D_i\) for \(i = 1,2 \equiv \) VEGF–VEGFR\(_i\) complexes on ECs and TCs). Note that in addition to binding and releasing VEGF, the rate of change of each receptor species is also affected by the creation of new unbound receptors as a result of cell division and the removal of receptors and complexes due to cell death. This ensures conservation of receptors and is described by the fourth term in the \(D_i\) equations and the fourth and fifth terms in the \(R_i\) equations. The last terms in each of the \(R_i\) equations represent the response to therapies that target the VEGF receptors.
1.2.1 Intracellular Bcl-2
Bcl-2 mRNA is constitutively expressed within both cell types and undergoes natural degradation. Activation of its receptors by VEGF leads to additional Bcl-2 synthesis in each cell type. Combining these processes, we obtain the following equations for Bcl-2 mRNA expression in ECs and TCs, as described in the main text:
1.2.2 Molecular-Level Variables Associated with Treatment
Below, we present the model equations for the effects of an anti-VEGF antibody, like avastin, an anti-VEGFR1 antibody-like IMC-18F1, and an anti-VEGFR2 antibody.
Drug Pharmacokinetics: Experimental evidence suggests a biphasic plasma concentration–time curve for avastin Lin et al. (1999). Consequently, a two-compartment model is proposed to govern the pharmacokinetics of all of the drugs we consider. Systemic circulation (together with well-vascularized organs) is taken to be the first compartment from where the drug may be eliminated or enter, in a reversible process, a second compartment which consists of tissues and organs with poor vascular perfusion. Combining these effects, the equations governing drug pharmacokinetics can be written as follows:
where \(X_{is}\) and \(X_{ip}\) (\(i = V, R1, R2\) for anti-VEGF, anti-VEGFR1, and anti-VEGFR2 therapy) represent the amount of drug in systemic circulation and the peripheral compartment, respectively. The dosing function in Eq. (43) represents the periodic administration of avastin and is taken to have the following form:
where \(D_0\) is the amount of drug delivered in each dose; \(t_i\), the timing of each dose; \(t_a\), the length of each injection; and n, the total number of doses.
Intratumoral Drug Dynamics: The tumor is assumed to reside in a pharmacokinetic compartment of its own. For simplicity, the rates of transfer of drug into and out of the tumor are taken to be the same as those for the peripheral compartment [Eq. (44)]. Further, the tumor is assumed to occupy a negligible volume compared to the volume of the animal; therefore, the extravasation of drug from the tumor into systemic circulation will not affect the amount of drug in the central compartment. Finally, avastin–VEGF complexes are assumed to undergo the same degradation as free VEGF in tissue. Combined together, these processes yield the following equations governing drug dynamics in the tumor space. Recall, \(X_{i}\) (\(i = V, R1, R2\) for the anti-VEGF, anti-VEGFR1 and anti-VEGFR2 therapy) represent free forms of each drug and \(C_{j}\) (\(j = AV, E1R1, T1R1, E2R2\)) represent the VEGFR1–anti-VEGFR1 antibody complexes on ECs/TCs and the VEGFR2–anti-VEGFR2 antibody complexes on ECs.
1.2.3 Receptor Conservation
In the above equations, \(R_{Ei}^t\), \(i=1,2\) and \(R_{T1}^t\) represent the VEGFRi and VEGFR1 receptor expression levels on endothelial and tumor cells, respectively. From equations (13), (35), (38) and (49), it follows that \(R_{E1}^tE \,=\, R_{E1}\,+\,D_{E1} \,+\, C_{E1R1}\), that is, the number of VEGFR1 per endothelial cell is conserved. Likewise, \(R_{E2}^tE \,=\, R_{E2}\,+\,D_{E2} \,+\, C_{E2R2}\) and \(R_{T1}^tT \,=\, R_{T1}\,+\,D_{T1} \,+\, C_{T1R1}\).
1.3 Parameters
A list of parameter values estimated from the literature for the in vitro and in vivo treatment models is provided in Table 5. We remark that the rates of forward reaction are typically expressed in units of “per time, per concentration.” However, in our model, all chemical species are expressed in terms of total quantity (fmol in the in vitro case and pmol in the in vivo case). Therefore, to preserve dimensional accuracy, the rates of forward reaction listed in Table 4 (and Table 1 of the main text) have been scaled by the volume of the experiment being simulated. For instance, the rate of forward reaction between avastin and VEGF has been determined experimentally to be 2.9794 (fmol/ml)\(^{-1}\) day\(^{-1}\) (or (pmol/\(\upmu \)l)\(^{-1}\) day\(^{-1}\)). Consequently, \(k_{VAb}^f\) is taken to be 2.9794/Vol\(_C\) fmol\(^{-1}\) day\(^{-1}\) when simulating in vitro experiments and 2.9794/Vol\(_T\) pmol\(^{-1}\) day\(^{-1}\) in the in vivo case. Here, \(Vol_C\) is the volume of the cell culture experiments in ml and \(\hbox {Vol}_T\), the volume of the tumor in \(\mu \)l. Note that \(Vol_T\) \(=\) (volume of 1 million ECs)\(\times E\) \(+\) (volume of 1 million TCs)\(\times T\) and the volume of 1 EC is 2.2\(\times 10^{-6}\) \(\mu \)l King et al. (2004) and that of 1 TC is 1\(\times 10^{-6}\) \(\mu \)l Monte (2009).
A list of parameter values for the in vivo model is provided in Table 6.
The best fit of the in vivo treatment model to avastin pharmacokinetic data reported in Lin et al. (1999) is shown in Fig. 9.
Model fit to avastin pharmacokinetic data reported in Lin et al. (1999)
1.4 Parameter Sensitivity
A local sensitivity analysis is carried out by varying the parameters associated with the vascular growth and response to treatment of in vivo tumor xenografts. These include: the VEGF-dependent rate of tumor cell proliferation, \(\nu _T\); the sensitivity of tumor cell proliferation to VEGFR1 receptor occupancy, \(\alpha _T\); the Bcl-2-modulated rate of tumor cell death, \(\delta _T\); the sensitivity of tumor cell death to Bcl-2 expression, \(\beta _T\); the VEGF-dependent rate of endothelial cell proliferation, \(\mu _E\); the sensitivity of endothelial cell proliferation to VEGFR2 receptor occupancy, \(\alpha _E\); the Bcl-2-modulated rate of endothelial cell death, \(\delta _E\); the sensitivity of endothelial cell death to Bcl-2 expression, \(\beta _E\); the VEGF expression rate of tumor cells, \(\nu _A\); and the sensitivity of tumor cells to hypoxia, \(\kappa _A\) [see equations (12)–(14) in the main text]. The two cases—control (no treatment) and treatment—are considered separately. In each case, the residual between simulated and experimental data (tumor volume time course) is computed as each parameter is varied from its baseline estimate, and the resulting residual is plotted as a function of the percent change in the value of parameter being varied. Given that vascular tumor growth is driven by hypoxia in our model, we also test the sensitivity of tumor oxygenation to the various parameters by plotting the maximum partial pressure of oxygen as a function of the percent change in the value of parameter being varied.
Local Sensitivity Analysis–Control
Figure 10a, c, e reveal that the model predictions of tumor xenograft size are sensitive to endothelial and tumor cell growth parameters, tumor cell death parameters, as well as tumor cell VEGF expression and sensitivity to hypoxia, with low values of \(\alpha _T\) and high values of \(\nu _A\) and \(\nu _T\) resulting in the most change. Since these simulations are without treatment, tumor xenograft size is predictably insensitive to parameters relating to endothelial cell death and tumor cell death. Figure 10b, d, f reveal that tumor oxygenation is most sensitive to high values of \(\mu _E\) and \(\alpha _T\) and low values of \(\alpha _E\), \(\nu _T\) and \(\nu _A\). Once again, cell death has little impact on tumor oxygenation.
Local sensitivity analysis for parameters relating to tumor xenograft growth in the absence of treatment. a, c, e The residual or Euclidean norm between simulated and experimental data (tumor volume time course, see Fig. 6a in main text) is plotted on the y-axis, and percentage variation of the parameters from their baseline values is plotted on the x-axis. b, d, f The predicted maximum partial pressure in the tumor over the time course of the simulation is plotted on the y-axis, and percentage variation of the parameters from their baseline values is plotted on the x-axis
Local Sensitivity Analysis–Treatment
Figure 11a, c, e reveal that the model predictions of tumor xenograft size under avastin therapy are sensitive to endothelial and tumor cell growth parameters, as well as tumor cell VEGF expression and sensitivity to hypoxia, with low values of \(\alpha _T\) and high values of \(\nu _A\) and \(\nu _T\) resulting in the most change. VEGF expression level per tumor cell (\(\nu _A\)) now emerges as the most influential parameter. Interestingly, although the simulations are sensitive to tumor cell death parameters, cellular proliferation rates are still more influential in comparison, indicating that the inhibition of cell proliferation—as opposed to inducing cell death—might be what drives the observed changes in xenograft volume in response to treatment with avastin. Figure 11b, d, f reveal that tumor oxygenation is sensitive to high values of \(\mu _E\), \(\alpha _T\), \(\delta _T\) and \(\beta _T\) and low values of \(\alpha _E\), \(\nu _T\) and \(\nu _A\). In contrast to the earlier case, the tumor cell death rate \(\delta _T\) emerges as the most influential parameter since increased tumor cell death could have the overall effect of increasing tumor oxygen levels.
Local sensitivity analysis for parameters relating to tumor xenograft growth under treatment with avastin. a, c, e The residual or Euclidean norm between simulated and experimental data (tumor volume time course, see Fig. 6a in main text) is plotted on the y-axis, and percentage variation of the parameters from their baseline values is plotted on the x-axis. b, d, f The predicted maximum partial pressure in the tumor over the time course of the simulation is plotted on the y-axis, and percentage variation of the parameters from their baseline values is plotted on the x-axis
Global Sensitivity Analysis
Although the above analysis provides some information on which parameters are more influential in our model, this analysis is local, with each parameter varied individually, keeping all others fixed. This approach suffers from the limitation that only a small fraction of parameter space is explored and does not take into account the simultaneous variation, and thus possible interactions, of model parameters. We therefore also conduct a global sensitivity analysis on the same set of parameters using the elementary effects method developed by Morris (1991) and implemented in MATLAB by Pianosi et al. (2015). An elementary effect is computed as follows. Consider a model with n independent input parameters \(x_i\), \(i=1,\ldots ,n\) with each parameter uncertainty interval divided into p equal intervals resulting in an n-dimensional p-level hyperspace. For any given set of parameter values, the elementary effect of the ith input factor on the output y is defined as:
Global sensitivity analysis for parameters relating to tumor xenograft growth in the absence of treatment. a Phase plane showing the mean versus standard deviation of elementary effects (EEs). b Bar graphs showing the corresponding global sensitivity indices for the various parameters when the output function is taken to be the residual or Euclidean norm between simulated and experimental data (tumor volume time course, see Fig. 6a in main text). c Phase plane showing the mean versus standard deviation of elementary effects (EEs). d Bar graphs showing the corresponding global sensitivity indices for the various parameters when the output function is taken to be the maximum partial pressure in the tumor over the time course of the simulation
where \(\Delta = p/(2(p-1))\). The distribution of elementary effects associated with \(x_i\) is then obtained by randomly sampling r different sets of parameters from the prescribed hyperspace. Thus, the Morris method depends not only on p, but also on the sampling number r. The sensitivity measures proposed by Morris are \(\mu _i\) and \(\sigma _i\)—the mean and the standard deviation, respectively, of the distribution of elementary effects for each \(x_i\). The mean \(\mu _i\) assesses the overall influence of one parameter on the output while the standard deviation \(\sigma _i\) assesses the parameter’s higher-order effects, such as interactions with other parameters or nonlinear effects on the output. For instance, a low value of \(\sigma _i\) indicates that the effect of \(x_i\) is almost independent of the values taken by the other factors. Elementary effects are typically represented graphically in the \((\mu _i,\sigma _i)\) plane, and the most influential parameters appear on the upper right corner.
An alternative and more quantitative measure to determine a parameter \(x_i\)’s sensitivity is its global index (Ciric et al. 2012), defined as:
Figure 12a, b reveal that in the control case, the output y—defined as the residual between simulated and experimental data (tumor volume time course)—is most sensitive to \(\alpha _T\) (tumor cell proliferation rate sensitivity to activated VEGFR1) and \(\mu _E\) (activated VEGFR2-mediated endothelial cell proliferation rate), followed by \(\nu _T\) (activated VEGFR1-mediated tumor cell proliferation rate), \(\nu _A\) (tumor cell VEGF expression level) and \(\alpha _E\) (endothelial cell proliferation rate sensitivity to activated VEGFR2). However, as shown in Fig. 12c, d, when the output is chosen as the maximum level of tumor oxygenation, the most influential parameters emerge as \(\alpha _T\) and \(\nu _A\), followed by \(\delta _T\) (tumor cell death rate), \(\alpha _E\) and \(\mu _E\). We remark that \(\delta _T\) has a high global sensitivity index even though locally the model is insensitive to it.
In the treatment case, Fig. 13a, b reveal that when the output is taken as the residual between simulated and experimental data, the most influential parameters remain \(\alpha _T\), \(\mu _E\) and \(\nu _T\) followed by \(\nu _A\) and \(\alpha _E\). However, as shown in Fig. 13c, d, when the output is chosen as the maximum level of tumor oxygenation, the most influential parameters emerge as \(\alpha _T\), \(\delta _T\) and \(\alpha _E\), followed by \(\nu _A\), \(\mu _E\), \(\nu _T\) and \(\delta _E\).
Global sensitivity analysis for parameters relating to tumor xenograft growth under treatment with avastin. a Phase plane showing the mean versus standard deviation of elementary effects (EEs). b Bar graphs showing the corresponding global sensitivity indices for the various parameters when the output function is taken to be the residual or Euclidean norm between simulated and experimental data (tumor volume time course, see Fig. 6a in main text). c Phase plane showing the mean versus standard deviation of elementary effects (EEs). d Bar graphs showing the corresponding global sensitivity indices for the various parameters when the output function is taken to be the maximum partial pressure in the tumor over the time course of the simulation
Rights and permissions
About this article
Cite this article
Jain, H., Jackson, T. Mathematical Modeling of Cellular Cross-Talk Between Endothelial and Tumor Cells Highlights Counterintuitive Effects of VEGF-Targeted Therapies. Bull Math Biol 80, 971–1016 (2018). https://doi.org/10.1007/s11538-017-0273-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-017-0273-6