Abstract
We implement an agent-based model for Clostridium difficile transmission in hospitals that accounts for several processes and individual factors including environmental and antibiotic heterogeneity in order to evaluate the efficacy of various control measures aimed at reducing environmental contamination and mitigating the effects of antibiotic use on transmission. In particular, we account for local contamination levels that contribute to the probability of colonization and we account for both the number and type of antibiotic treatments given to patients. Simulations illustrate the relative efficacy of several strategies for the reduction of nosocomial colonizations and nosocomial diseases.


Similar content being viewed by others
References
Bartlett JG (2002) Antibiotic-associated diarrhea. N Engl J Med 346(5):334–339
Bignardi G (1998) Risk factors for Clostridium difficile infection. J Hosp Infect 40(1):1–15
Brauer F (2015) Some simple nosocomial disease transmission models. Bull Math Biol 77(3):460–469
Codella J, Safdar N, Heffernan R, Alagoz O (2015) An agent-based simulation model for Clostridium difficile infection control. Med Decis Mak 35(2):211–229
Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH (2010) Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 31(5):431–455
Curry SR, Muto CA, Schlackman JL, Pasculle AW, Shutt KA, Marsh JW, Harrison LH (2013) Use of multilocus variable number of tandem repeats analysis genotyping to determine the role of asymptomatic carriers in Clostridium difficile transmission. Clin Infect Dis 57(8):1094–1102
D’Agata EM, Magal P, Olivier D, Ruan S, Webb GF (2007) Modeling antibiotic resistance in hospitals: the impact of minimizing treatment duration. J Theor Biol 249(3):487–499
Dancer S, Kirkpatrick P, Corcoran D, Christison F, Farmer D, Robertson C (2013) Approaching zero: temporal effects of a restrictive antibiotic policy on hospital-acquired Clostridium difficile, extended-spectrum \(\beta \)-lactamase-producing coliforms and meticillin-resistant Staphylococcus aureus. Int J Antimicrob Agents 41(2):137–142
Donskey CJ (2010) Preventing transmission of Clostridium difficile: Is the answer blowing in the wind? Clin Infect Dis 50(11):1458–1461
Dubberke ER, Gerding DN, Classen D, Arias KM, Kelly CP, Deverick MC, Anderson J, Burstin H, Calfee DP, Coffin SE, Fraser V, Griffin FA, Gross P, Kaye KS, Klompas M, Lo E, Marschall J, Mermel LA, Nicolle L, Pegues DA, Perl TM, Saint S, Salgado CD, Weinstein RA, Wise R, Yokoe DS (2008) Strategies to prevent Clostridium difficile infections in acute care hospitals. Infect Control Hosp Epidemiol 29(S1):S81–S92
Dubberke ER, Carling PM, Carrico R, Donskey CJ, Loo VG, McDonald LC, Maragakis LL, Sandora TJ, Weber DJ, Yokoe DS, Gerding DN (2014) Strategies to prevent Clostridium difficile infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 35(6):628–645
Dubberke ER, Olsen MA (2012) Burden of Clostridium difficile on the healthcare system. Clin Infect Dis 55(suppl 2):S88–S92
Feazel LM, Malhotra A, Perencevich EN, Kaboli P, Diekema DJ, Schweizer ML (2014) Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother 69(7):1748–1754
Gerding DN, Muto CA, Owens RC (2008) Measures to control and prevent Clostridium difficile infection. Clin Infect Dis 46(Supplement 1):S43–S49
Grimm V, Berger U, DeAngelis DL, Polhill JG, Giske J, Railsback SF (2010) The ODD protocol: a review and first update. Ecol Model 221(23):2760–2768
Hsieh Y-H, Liu J, Tzeng Y-H, Wu J (2014) Impact of visitors and hospital staff on nosocomial transmission and spread to community. J Theor Biol 356:20–29
Hsu J, Abad C, Dinh M, Safdar N (2010) Prevention of endemic healthcare-associated Clostridium difficile infection: reviewing the evidence. Am J Gastroenterol 105(11):2327–2339
Lanzas C, Dubberke ER, Lu Z, Reske KA, Grohn Y (2011) Epidemiological model for Clostridium difficile transmission in healthcare settings. Infect Control Hosp Epidemiol 32(06):553–561
Lanzas CP, Dubberke ER (2014) Effectiveness of screening hospital admissions to detect asymptomatic carriers of Clostridium difficile: a modeling evaluation. Infect Control Hosp Epidemiol 35(8):1043–1050
Leffler DA, Lamont JT (2015) Clostridium difficile infection. N Engl J Med 372(16):1539–1548
Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC (2015) Burden of Clostridium difficile infection in the United States. N Engl J Med 372(9):825–834
McFarland LV (2008) Update on the changing epidemiology of Clostridium difficile-associated disease. Nat Clin Pract Gastroenterol Hepatol 5(1):40–48
McMaster-Baxter N L, Musher D M (2007) Clostridium difficile: recent epidemiologic findings and advances in therapy. Pharmacother J Hum Pharmacol Drug Therapy 27(7):1029–1039
Otten AM, Reid-Smith RJ, Fazil A, Weese JS (2010) Disease transmission model for community-associated Clostridium difficile infection. Epidemiol Infect 138:907–914
Owens RC, Donskey CJ, Gaynes RP, Loo VG, Muto CA (2008) Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis 46:S19–31
Planche T, Aghaizu A, Holliman R, Riley P, Poloniecki J, Breathnach A, Krishna S (2008) Diagnosis of Clostridium difficile infection by toxin detection kits: a systematic review. Lancet Infect Dis 8(12):777–784
Rubin MA, Jones M, Leecaster M, Khader K, Ray W, Huttner A, Huttner B, Toth D, Sablay T, Borotkanics RJ, Gerding DN, Samore MH (2013) A simulation-based assessment of strategies to control Clostridium difficile transmission and infection. PLoS One 8(11):e80671
Rupnik M, Wilcox MH, Gerding DN (2009) Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol 7(7):526–536
Slimings C, Riley TV (2014) Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother 69(4):881–891
Steiner C, Barrett M, Terrel L (2012) HCUP projections:Clostridium difficile hospitalizations 2011 to 2012. HCUP Projections Report # 2012-01
Sullivan A, Edlund C, Nord CE (2001) Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis 1(2):101–114
Talpaert MJ, Gopal Rao G, Cooper BS, Wade P (2011) Impact of guidelines and enhanced antibiotic stewardship on reducing broad-spectrum antibiotic usage and its effect on incidence of Clostridium difficile infection. J Antimicrob Chemother 66(9):2168–2174
U.S. Department of Health and Human Services (2013) National action plan to prevent health care-associated infections: road map to elimination. Technical report
Webb G, Blaser MJ, Zhu H, Ardal S, Wu J (2004) Critical role of nosocomial transmission in the toronto sars outbreak. Math Biosci Eng 1(1):1–13
Yahdi M, Abdelmageed S, Lowden J, Tannenbaum L (2012) Vancomycin-resistent enterococci colonization-infection model: parameter impacts and outbreak risks. J Biol Dyn 6(2):645
Acknowledgments
The work of all the authors was partially supported by the National Institute of Mathematical and Biological Synthesis (NIMBioS), an Institute sponsored by National Science Foundation, the US Department of Homeland Security, and the US Department Agriculture through NSF Awards #EF-0832858 and #DBI-1300426, with additional support from University of Tennessee, Knoxville. This work was also supported by the joint NSF/NIGMS Mathematical Biology Program through NIH award R01GM113239. Lenhart’s work is also partially supported by the University of Tennessee Center for Business and Economic Research.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: ODD Protocol: Overview
1.1 Purpose
We present an ABM that simulates the transmission of C. difficile in healthcare settings in order to evaluate the efficacy of various control measures (e.g., antimicrobial and environmental stewardship) in reducing the nosocomial incidence of colonization and infection.
1.2 Input Data
The model layout and agent behaviors as well as rate parameters and initial conditions come from published studies as well as previously collected epidemiological data from Barnes-Jewish Hospital in St. Louis, Missouri. These data are for a retrospective cohort of 11,046 admissions in medicine wards at a large tertiary care hospital, which included laboratory-confirmed cases of CDI, admission, discharge, and confirmed laboratory dates, and antimicrobial exposures. Summary data from this set were provided by Lanzas and further description of these can be found in Lanzas et al. (2011).
Control measures for reducing nosocomial colonization and infection are model inputs. The level of contamination is one factor contributing to the probability of a susceptible patient becoming colonized. We regard effective cleaning to be cleaning that reduces the contamination level of a ward room (‘Cleaning’ of “Appendix 2”). The probability that a vacant room will be effectively cleaned (terminal cleaning) is a global variable. Changing the value of this probability is a control input for the model representing different cleaning programs.
Another control strategy of the model is to reduce the overall number of antibiotic treatments by a certain proportion. This is implemented in the following way. Let q be the proportion reduction to be implemented. Of all the patients that were assigned to receive a treatment each half-day according to the half-daily probability of receiving an antibiotic (see Table 5), only \(1-q\) of them will now actually receive a treatment.
Alternatively, one may change the relative proportions of the types of antibiotic treatments that are prescribed. Each time an antibiotic treatment is prescribed, there is a probability that it will be low, high, or very-high risk with respect to CDI. The baseline values for these probabilities were taken from the data and are 0.4, 0.26 and 0.34, respectively (see Table 5). These proportions can be changed in accordance with a specified stewardship program.
1.3 Entities, State Variables, and Scales
The model has two kinds of entities: hospital patients and rooms. Individual rooms are classified by two state variables. A room has a certain level of contamination—low, medium, or high—and a room either is or is not occupied by a patient under quarantine. Symptomatic patients are placed in quarantine or isolation for the purpose of implementing contact precautions that decrease the environmental bioburden associated with diarrhea (Gerding et al. 2008). While healthcare workers are not agents of this model, their effect as vectors for disease transmission is implicit in the fact that ward-level contamination levels contribute to patient colonization. That is, the probability that a susceptible patient in a particular ward room will become colonized depends in part on the contamination level of the entire ward. We regard the contamination level of a hospital ward a reasonable measure of the scope of a healthcare worker’s interaction with patients.
Patients are classified by several state variables. These are summarized in Table 4. With respect to C. difficile infection (CDI), a patient can be resistant to colonization, susceptible to colonization, colonized, or diseased. Each patient is assigned a (hospital) length of stay (LOS) and the patient’s time since being admitted is tracked. The time since a patient’s current disease status is tracked. If a patient goes on an antibiotic, the time since beginning the antibiotic treatment is tracked as well as the level of CDI risk associated with the antibiotic with which they are being treated. This model considers three such levels of risk—low, high, and very high risk. The number of antibiotics a patient has received during hospitalization is also tracked. Colonized patients either will or will not mount an immune response against C. difficile. Those who will not are said to be immunocompromised. Diseased patients either will or will not be identified as diseased upon screening and those that are treated for the disease either will or will not be treated successfully.
We here describe the model’s global variables. A summary of these global variables and their values is given in Table 5. The occupancy level for the hospital is a global variable set to 0.85. The probability of a patient being resistant upon admission is 0.58, while the probability of being diseased upon admission is 0.01. The probability of being susceptible upon admission is chosen randomly from a uniform distribution ranging from 0.21 to 0.40. This value will fix the corresponding probability of being colonized upon admission which will range between 0.01 and 0.20. The probability that a colonized patient will be immunocompromised is a global variable. The baseline value for this variable is 0.10. There is a half-daily probability that a susceptible patient or a colonized patient that is not immunocompromised will regain resistance. The minimum such probability is a global variable, and its value is 0.2. For a full description see ‘Update Disease Status’ of “Appendix 2”. There is a half-daily probability that a patient will begin an antibiotic treatment. The baseline value for this variable is 0.27. This value was chosen so that simulation outputs pertaining to overall number of antibiotic treatments per patient reflect the hospital dataset. Each time an antibiotic treatment is prescribed, there is a probability that it will be low, high, or very-high risk with respect to CDI. The baseline values for these probabilities were taken from the data and are 0.4, 0.26 and 0.34, respectively. The odds ratio values for high and very-high-risk antibiotics are global variables and are given by OR\(_\mathrm{h}=4\) and OR\(_\mathrm{vh}=8\), respectively. For a full description see ‘Assign Probability of Becoming Colonized’ of “ Appendix 2”. The half-daily probability of a susceptible patient becoming colonized given that they are being treated with a low-risk antibiotic in a highly contaminated environment, denoted by \(p_\mathrm{l}^\mathrm{h}\), is a global variable and its value is 0.15. Again, for a full description see ‘Assign Probability of Becoming Colonized’ of “ Appendix 2”. Each time a room is cleaned, there is a probability that it will be cleaned effectively. For a description of what this means see ‘Cleaning’ of “ Appendix 2”. A baseline value of 0.5 was assigned to this variable under the simple assumption that different cleaning measures could be more or less effective at reducing the level of contamination. In our model it is assumed that patients with CDI are also symptomatic. Once a patient becomes diseased and, thus, symptomatic, they are screened for CDI. The sensitivity (0.91) of this test is a global variable as well as the turnover time (2 half-days) for this test (Planche et al. 2008). When a patient is treated for CDI, there is a probability (0.8) that the treatment will be successful.
The spatial scale of the model is one hospital consisting of six medical wards, each containing thirty-five rooms. This is reflective of the hospital from which the previously described dataset came. In this model we assume that at most one patient can occupy a room. The time step is one half-day and the temporal extent of the simulation is 1 year.
1.4 Process Overview and Scheduling
The following process takes place each time step (half-day). Time-tracking characteristics for patients are updated. New patients are admitted, the contamination of the environment is updated, the patients progress with respect to their infection status, patients are discharged, and vacant rooms are cleaned.
1.5 Initialization
The model hospital is initially populated with patients who have various hospital and pre-hospital histories with respect to each of the patient variables listed in Table 4. The occupancy level is a global variable set to 0.85 and is kept constant. The environment is then initialized (see ‘Update Contamination Status’ of “Appendix 2” for details). As the patients from this initial population are discharged, new patients are admitted. The hospital-history of these new patients more accurately reflects the processes of the model. In order for the initial hospital population not to have influence on model outputs, the simulation runs 200 time steps before recording outputs.
Appendix 2: ODD Protocol: Submodels
This section describes in detail the subroutines that make up the main process.
1.1 Update Time Characteristics
Patients’ time since admission and, with one exception, time since current disease status are updated. For resistant patients, the time since current disease status is not tracked. This is because the value of this state variable is only relevant for the progression of patients who are susceptible, colonized or diseased. Unlike them, a patient who is resistant will remain resistant until and unless they receive an antibiotic at which time they become susceptible to colonization.
Susceptible patients and colonized patients who are not immunocompromised have their time since beginning antibiotic treatment updated. Colonized patients who are immunocompromised have their time until diseased updated. Three classes of diseased patients are considered here. Those who have been screened successfully but have not yet reached the turnaround time have their time since screening updated. The turnaround time for a screening is the time between administering the test and receiving the results. Those who have been screened unsuccessfully but have not yet reached the turnaround time also have their time since screening updated. In this way, they will not be screened again until at least the turnaround time has passed. Finally, those who have been screened successfully and have begun treatment have their time since beginning treatment updated.
1.2 Admission
Each time step, a number of patients are admitted. This is referred to as an admission class. The same number of patients is admitted as were just discharged. This is done to assure consistency, so that the number of patients to be admitted does not exceed the number of vacant rooms. Moreover, the number of patients being discharged varies significantly each time step since it is ultimately based on the patient’s varying lengths of stay and times since admission.
For each admission class, the probability of a patient being resistant upon admission is 0.58, while the probability of being diseased upon admission is 0.01 (Lanzas et al. 2011). However, for each admission class, at each time step, the probability of being susceptible upon admission is chosen randomly from a uniform distribution ranging from 0.21 to 0.40. This value will fix the corresponding probability of being colonized upon admission which will range between 0.01 and 0.20.
Each patient is randomly admitted to a vacant room and their time since admission is initialized. They are then assigned a disease status according to the above probabilities as well as a length of stay based on the hospital dataset. The procedure for this assignment is described in detail in ‘Length of Stay’ of “Appendix 2”.
Susceptible patients are given an antibiotic history since patients become susceptible to colonization via the disruption of the gut microbiota caused by antibiotic treatment. First, a particular type of antibiotic is assigned according to the treatment length, time until flora recovery, and the risk level vis à vis CDI associated with this antibiotic. This procedure is described in ‘Antibiotic Assignment’ of “Appendix 2”. Second, a time since beginning antibiotic treatment is assigned. This is a random integer drawn from a uniform distribution ranging from 0 to an upper limit defined as the sum of the treatment length (14 half-days) and time until microbiota recovery (28 half-days for low and high-risk antibiotic, 70 half-days for very high-risk antibiotic). That is, we regard a patient as susceptible to colonization from the moment they begin an antibiotic treatment and they can remain susceptible as long as their gut microbiota is not normal. Finally, the patient is assigned a time since becoming susceptible. In this case, it is precisely the time since they began antibiotic treatment.
Colonized patients either will or will not mount an immune response and so are characterized as one or the other according the global variable for the probability that a colonized patient is immunocompromised. Patients who are not immunocompromised are given an antibiotic history. First, an antibiotic is assigned in the same way as described above for susceptible patients. Second, a time since beginning antibiotic treatment is assigned; again, in the same way as for susceptible patients. Lastly, they are assigned a time since becoming colonized. A patient may have become colonized at any time since they began antibiotic treatment and, thus, this variable is assigned a uniform random integer between 0 and the time since they began treatment.
If the colonized patient is immunocompromised, they will become diseased at a certain point in time that must be assigned. First, an antibiotic history is assigned. In this case, what matters most is the risk level associated with their antibiotic assignment. Next, the length of the incubation period is determined. The incubation period depends on the risk level associated with their antibiotic assignment. For each level of antibiotic risk, there is a pair of global values for the minimum and maximum length of the incubation period. Baseline values for these pairs are (20,60), (14,40), and (8,20) for low, high, and very high-risk antibiotics, respectively. A random integer from a uniform distribution of integers in the appropriate range is assigned as the length of the incubation period. The patient’s time until becoming diseased is assigned as a random integer greater than or equal to 0 but less than the incubation period. The time since current disease status in this case is equal to the length of the incubation period minus the time until becoming diseased.
Finally, for patients who are diseased upon admission, it is decided if the initial screening will be successful in identifying them as diseased according to the global variable for the sensitivity of the test and it is also decided if treatment will be successful according the global variable for this probability. The baseline values for the sensitivity and probability of successful treatment are 0.91 (Planche et al. 2008) and 0.8 (McFarland 2008), respectively. Patients who will be identified as diseased due to successful screening are assigned a time since the successful screen. In this way, they will be identified when that time reaches the turnaround time for the test. The baseline value for the turnaround is 2 half-days (Planche et al. 2008). At that point the patient will be quarantined and treatment will begin as described in ‘Quarantine and Treat’ of “Appendix 2”. Diseased patients who are unsuccessfully screened will not be tested again until after the turnaround time for the test and so are assigned a time since the unsuccessful screening.
1.3 Update Contamination Status
After a class of patients is admitted, the environment is updated. Each room has a contamination status of clean, contaminated, or very contaminated represented by the values 0, 1, and 2, respectively. During an update, the contamination status of a room occupied by a colonized patient is set to 1, while the contamination status of a room occupied by a diseased patient is set to 2. This value is affected by the cleaning procedure ‘Cleaning’ of “Appendix 2”).
The ward-level contamination is the sum of the contamination status values of all the rooms in the same ward. Since each ward has 35 rooms, each with a maximum contamination value of 2, this will be an integer between 0 and 70. This sum excludes the contamination values of those rooms that contain a quarantined patient. Thus, our model assumes that quarantine is 100 % effective. This does not effect, however, the uncertain level of cleaning the room will receive upon the discharge of the quarantined patient. This value will be used in determining the probability that a susceptible patient will become colonized ‘Assign Probability of Becoming Colonized’ of “Appendix 2”. While healthcare workers are not agents of this model, their effect as vectors for disease transmission is implicit in the fact that ward-level contamination levels contribute to patient colonization. That is, the probability that a susceptible patient in a particular ward room will become colonized depends in part on the contamination level of the entire ward. We regard a hospital ward a reasonable measure of the scope of a healthcare worker’s interaction with patients. Therefore, the contamination level of the ward, rather than that of the individual patient room, contributes to the probability of becoming colonized.
1.4 Update Disease Status
Each half-day there is a probability that a patient will begin an antibiotic treatment—even if they are currently being treated with antibiotics. This model parameter was chosen so that simulation outputs pertaining to overall number of antibiotic treatments per patient reflect the hospital dataset. One of the model control strategies is to reduce the overall number of treatments by a certain proportion. This is implemented in the following way. Of all the patients that were assigned to receive a treatment each half-day according to the probability just described, only a proportion of them will now actually receive a treatment.
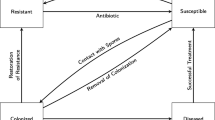
The transitions described in this section are illustrated by the diagram in Fig. 3. If a resistant patient goes on an antibiotic, they become susceptible and an antibiotic is assigned. The time since beginning antibiotic and time since current disease status are set to 0. A new length of stay is selected for this patient according the new disease status. If it is longer than the patient’s current length of stay, then the patient’s length of stay is changed to this value.
Each half-day, there is a probability that a susceptible patient will regain resistance. This probability is a logistic function of the time since they began their most recent antibiotic treatment. In particular, let t be the time since the patient began antibiotic treatment and let T be the sum of the treatment length and the associated time until a normal microbiota is restored. Then, the probability, p, of regaining resistance is given by
where \(p_{\min }=0.2\) is the minimum probability of regaining resistance and the parameter value 12 determines the steepness of the logistic curve.
If they do not regain resistance, there is a probability they will receive an additional antibiotic as described above. Then, there is a probability they will become colonized (‘Assign Probability of Becoming Colonized’ of “ Appendix 2”). If they do become colonized, they are designated as either immunocompromised or not according to the global variable for the probability of being immunocompromised. If the now colonized patient is immunocompromised, then an incubation period is assigned as before. This value determines when they will become diseased. Furthermore, the number of nosocomial colonizations is tracked.
Similar to susceptible patients, colonized patients who will mount an immune response can regain resistance. If they do not regain resistance, there is a chance they will receive an additional antibiotic; this is also the case for those patients who will not mount an immune response. For these patients, if their incubation period is over, they become diseased. If that happens, they will be screened at the next time step (because they are now symptomatic) and it is determined whether the screening will be successful in identifying them as diseased according to the global variable for sensitivity. It is also decided if the subsequent treatment will be successful according the global variable for this probability. The number of nosocomial diseases is tracked.
A diseased patient is quarantined and treatment is begun (‘Quarantine and Treat’ of “ Appendix 2”) if they have been identified; that is, if they were successfully screened and they have reached the turnaround time. A diseased patient who has not been identified as diseased is re-screened if the turnover time since the unsuccessful screening has been reached. Diseased patients that have completed a successful treatment become susceptible.
1.5 Discharge Patients
Patients are discharged if their length-of-stay variable is the same value as their time-since-admission variable. The current disease status, disease status at admission and the number of antibiotics received during their stay are tallied for each discharged patient.
1.6 Cleaning
After a patient is discharged, the vacant room is cleaned. If the room has contamination status 2 or 1, then there is a probability that cleaning will result in a new contamination status of 1 or 0, respectively. The probability of effective cleaning is a global variable. A baseline value of 0.5 was assigned to this variable under the simple assumption that different cleaning measures could be more or less effective at reducing the level of contamination. Rooms are subject to terminal cleaning once the patient is discharge, but additional efforts on daily or terminal cleaning may be recommended if there is an outbreak or hyperendemicity (incidence above observed on similar facilities). This additional level of cleaning can be incorporated in the model by modifying the contamination status according patient status if cleaning targets rooms with diseased patients or the probability of effective cleaning if additional cleaning is applied to the whole ward.
1.7 Antibiotic Assignment
When a patient goes on an antibiotic, there is a probability that it will be low, high, or very high-risk with respect to CDI. Antibiotics disrupt the normal gut microbiota. The degree of disruption and the subsequent risk of C. difficile colonization depends on the spectrum of the antibiotic. Antibiotics were categorized in these three categories (low, high, and very high-risk) based on epidemiological studies reporting the association of type of antibiotic and C. difficile. There is a treatment length and time to normal flora associated with each class. The risk level is one factor in determining the probability of being colonized.
1.8 Assign Probability of Becoming Colonized
The half-daily probability of a susceptible patient becoming colonized depends on both the local, ward-level environment and the risk level associated with the antibiotic they are receiving.
The environment is classified as low, medium, or high contamination. These classifications are determined by the typical range and variance of ward-level contamination values for the simulations of this model.
The probabilities associated with different antibiotic risk levels (see ‘Antibiotic Assignment’ of “ Appendix 2”) are determined using odds ratios. The odds ratio (OR) of interest in this case is a measure of the association between an exposure to antibiotic treatment and the outcome of becoming colonized by C. difficile. Studies have quantified the odds ratios for infection risk assigned to specific antibiotics (Bignardi 1998; Feazel et al. 2014; Slimings and Riley 2014). In our model, the OR represents the odds that a patient will become colonized if they have been given a high or very high-risk antibiotic, compared to the odds of becoming colonized when given a low-risk antibiotic. These are global variables in the model.
Let p be the half-daily probability of a susceptible patient becoming colonized given that they are being treated with a low-risk antibiotic. In general, we can use p together with a known odds ratio value associated with a particular antibiotic to find the half-daily probability of the patient becoming colonized given they are being treated with that particular antibiotic. Let OR\(_A\) be the odds ratio associated with antibiotic A and let \(p_A\) be the half-daily probability of the patient becoming colonized given they are being treated with antibiotic A. Then the relationship
implies
Odds ratio values for the categories “high risk” and “very-high risk” are based on the odds ratio values for the individual antibiotics in these categories (Bignardi 1998; Feazel et al. 2014; Slimings and Riley 2014) as well as information from the hospital dataset regarding the relative proportion of the number of treatments for each specific antibiotic to the overall number of antibiotic treatments. For each category, a weighted average was calculated using the odds ratio values for each specific antibiotic in the category and the corresponding number of these treatments from the hospital dataset. In this way, we estimated the odds ratio values for high and very-high-risk antibiotics to be \(\hbox {OR}_\mathrm{h}=4\) and \(\hbox {OR}_\mathrm{vh}=8\), respectively.
Since there is also an environmental component to colonization, we will have half-daily probabilities for each of the nine combinations from the three environmental contamination levels and the three antibiotic risk categories; see Table 6. Let \(p_\mathrm{l}^\mathrm{h}\) be the half-daily probability of a susceptible patient becoming colonized given that they are being treated with a low-risk antibiotic in a highly contaminated environment. Here the subscript, \(\ell \), refers to the risk level associated with the antibiotic and stands for “low”. The superscript, h, refers to the level of ward contamination and stands for “high”. Table 6 indicates the values for each of the 9 combinations and how they are calculated.
First a value is assigned to \(p_\mathrm{l}^\mathrm{h}\). The value 0.15 for \(p_\mathrm{l}^\mathrm{h}\) was chosen by calibration to match the proportion of nosocomial infections from the data. From this value and the values for \(\hbox {OR}_\mathrm{h}\) and \(\hbox {OR}_\mathrm{vh}\), we calculate the other two probabilities for a highly contaminated environment, \(p_\mathrm{h}^\mathrm{h}\) and \(p_\mathrm{vh}^\mathrm{h}\). Next, we calculate the probability, \(p_\mathrm{l}^{\ell }\), of a susceptible patient becoming colonized given that they are being treated with a low-risk antibiotic in a low contamination environment. We do this by scaling down by a certain factor, q: \(p_\mathrm{l}^{\ell }=q\cdot p_\mathrm{l}^\mathrm{h}\). This factor is a measure of the relative contribution of the environment to the colonization of a susceptible patient. For the simulations we assigned the value 0.5 to the factor q. The other two probabilities for a low contamination environment are then calculated similar to above. Finally, we take \(p_\mathrm{l}^\mathrm{m}\) to be the average of \(p_\mathrm{l}^{\ell }\) and \(p_\mathrm{l}^\mathrm{h}\) and from this calculate the other two probabilities for a medium contamination environment.
1.9 Quarantine and Treat
In this model, quarantined means that the contamination level of their room does not contribute to the overall ward-level contamination. This models the effect of isolation and contact precautions that decrease the environmental bioburden associated with diarrhea (Gerding et al. 2008). When a patient is quarantined due to CDI, they begin treatment for CDI. This means they will be assigned an antibiotic ‘Antibiotic Assignment’ of Appendix 2” and the time since beginning treatment is initialized.
1.10 Length of Stay
Patients are assigned a length of stay upon admission according to their disease status. This half-daily value is assigned by resampling summary data from the hospital dataset. In particular, given a patient’s disease status, their length of stay is assigned by resampling from the dataset (from Barnes-Jewish Hospital) generated by the length of stay data for patients with that disease status. The range of the values for the length of stay in half-days for resistant, susceptible, and diseased patients are [0, 32], [0, 68] and [0, 160], respectively. The range for colonized patients is the same as that for susceptible patients.
Rights and permissions
About this article
Cite this article
Bintz, J., Lenhart, S. & Lanzas, C. Antimicrobial Stewardship and Environmental Decontamination for the Control of Clostridium difficile Transmission in Healthcare Settings. Bull Math Biol 79, 36–62 (2017). https://doi.org/10.1007/s11538-016-0224-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-016-0224-7