Abstract
Several studies suggest that one possible cause of impaired wound healing is failed or insufficient lymphangiogenesis, that is the formation of new lymphatic capillaries. Although many mathematical models have been developed to describe the formation of blood capillaries (angiogenesis), very few have been proposed for the regeneration of the lymphatic network. Lymphangiogenesis is a markedly different process from angiogenesis, occurring at different times and in response to different chemical stimuli. Two main hypotheses have been proposed: (1) lymphatic capillaries sprout from existing interrupted ones at the edge of the wound in analogy to the blood angiogenesis case and (2) lymphatic endothelial cells first pool in the wound region following the lymph flow and then, once sufficiently populated, start to form a network. Here, we present two PDE models describing lymphangiogenesis according to these two different hypotheses. Further, we include the effect of advection due to interstitial flow and lymph flow coming from open capillaries. The variables represent different cell densities and growth factor concentrations, and where possible the parameters are estimated from biological data. The models are then solved numerically and the results are compared with the available biological literature.









Similar content being viewed by others
Notes
An alternative approach would be to consider fibroblasts instead of capillaries here, but the introduction of a new variable and consequently a new equation does not seem to be worthwhile, since capillary presence is a good indication of the healing state of the wound.
References
Adams RH, Alitalo K (2007) Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8(6):464–478
Ambrose CT (2006) Immunology’s first priority dispute-an account of the 17th-century Rudbeck–Bartholin feud. Cell Immunol 242(1):1–8
Asai J, Takenaka H, Hirakawa S, Sakabe J, Hagura A, Kishimoto S, Maruyama K, Kajiya K, Kinoshita S, Tokura Y, Katoh N (2012) Topical simvastatin accelerates wound healing in diabetes by enhancing angiogenesis and lymphangiogenesis. Am J Pathol 181(6):2217–2224
Barkefors I, Le Jan S, Jakobsson L, Hejll E, Carlson G, Johansson H, Jarvius J, Park JW, Li Jeon N, Kreuger J (2008) Endothelial cell migration in stable gradients of vascular endothelial growth factor A and fibroblast growth factor 2: effects on chemotaxis and chemokinesis. J Biol Chem 283(20):13905–13912
Benest AV, Harper SJ, Herttuala SY, Alitalo K, Bates DO (2008) VEGF-C induced angiogenesis preferentially occurs at a distance from lymphangiogenesis. Cardiovasc Res 78(2):315–323
Bernatchez PN, Soker S, Sirois MG (1999) Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J Biol Chem 274(43):31047–31054
Bianchi A, Painter KJ, Sherratt JA (2015) A mathematical model for lymphangiogenesis in normal and diabetic wounds. J Theor Biol 383:61–86
Boardman KC, Swartz MA (2003) Interstitial flow as a guide for lymphangiogenesis. Circ Res 92:801–808
Boulton R, Woodman A, Calnan D, Selden C, Tam F, Hodgson H (1997) Nonparenchymal cells from regenerating rat liver generate interleukin-1alpha and -1beta: a mechanism of negative regulation of hepatocyte proliferation. Hepatology 26(1):49–58
Brem H, Tomic-Canic M (2007) Cellular and molecular basis of wound healing in diabetes. J Clin Invest 117(5):1219–1222
Brown DR (1999) Dependence of neurones on astrocytes in a coculture system renders neurones sensitive to transforming growth factor \(\beta \)1-induced glutamate toxicity. J Neurochem 72(3):943–953
Byrne HM, Chaplain MAJ (1995) Explicit solutions of a simplified model of capillary sprout growth during tumour angiogenesis. Appl Math Lett 8(5):71–76
Byrne HM, Chaplain MAJ, Evans DL, Hopkinson I (2000) Mathematical modelling of angiogenesis in wound healing: comparison of theory and experiment. J Theor Med 2(3):175–197
Cao C, Lawrence DA, Strickland DK, Zhang L (2005) A specific role of integrin Mac-1 in accelerated macrophage efflux to the lymphatics. Blood 106(9):3234–3241
Cao Y (2005) Opinion: emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer 5(9):735–743
Castiglioni A (1947) A history of medicine. Alfred A. Knopf, New York
Chaplain MAJ, Anderson ARA (1999) Modelling the growth and form of capillary networks. In: On growth and form: spatio-temporal pattern formation in biology, Wiley, pp 225–250
Chaplain MAJ, McDougall SR, Anderson ARA (2006) Mathematical modeling of tumor-induced angiogenesis. Annu Rev Biomed Eng 8:233–257
Cho CH, Sung HK, Kim KT, Cheon HG, Oh GT, Hong HJ, Yoo OJ, Koh GY (2006) COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. PNAS 103(13):4946–4951
Choi I, Lee S, Hong YK (2012) The new era of the lymphatic system: no longer secondary to the blood vascular system. Cold Spring Harb Perspect Med 2(4):a006,445
Christiansen A, Detmar M (2011) Lymphangiogenesis and cancer. Genes Cancer 2(12):1146–1158
Cobbold CA, Sherratt JA (2000) Mathematical modelling of nitric oxide activity in wound healing can explain keloid and hypertrophic scarring. J Theor Biol 204:257–288
De Crescenzo G, Grothe S, Zwaagstra J, Tsang M, O’Connor-McCourt MD (2001) Real-time monitoring of the interactions of transforming growth factor-\(\beta \) (TGF-\(\beta \)) isoforms with latency-associated protein and the ectodomains of the TGF-\(\beta \) type II and III receptors reveals different kinetic models and stoichiometries of binding. J Biol Chem 276(32):29632–29643
Dodson JF (1924-1925) Herophilus of alexandria. Proc R Soc Med 18:19–32
Douglas JF, Gasiorek JM, Swaffield JA, Jack LB (2005) Fluid mechanics, 5th edn. Prentice Hall, Upper Saddle River
Drew P, Posnett J, Rusling L (2007) The cost of wound care for a local population in England. Int Wound J 4(2):149–155
Edelstein L (1982) The propagation of fungal colonies: a model for tissue growth. J Theor Biol 98:679–701
Farrell BE, Daniele RP, Lauffenburger DA (1990) Quantitative relationships between single-cell and cell-population model parameters for chemosensory migration responses of alveolar macrophages to C5a. Cell Motil Cytoskelet 16(4):279–293
Fischer M, Costanzo U, Hoffmann U, Bollinger A, Franzeck UK (1997) Flow velocity of cutaneous lymphatic capillaries in patients with primary lymphedema. Int J Microcirc Clin Exp 17(3):143–149
Fischer M, Franzeck UK, Herrig I, Costanzo U, Wen S, Schiesser M, Hoffmann U, Bollinger A (1996) Flow velocity of single lymphatic capillaries in human skin. Am J Physiol 270(1 Pt 2):H358–H363
Flegg JA, Byrne HM, Flegg MB, McElwain DLS (2012) Wound healing angiogenesis: the clinical implications of a simple mathematical model. J Theor Biol 300:309–316
Flegg JA, Menon SN, Maini PK, McElwain DLS (2015) On the mathematical modeling of wound healing angiogenesis in skin as a reaction-transport process. Front Physiol 6:262. doi:10.3389/fphys.2015.00262
Fleury ME, Boardman KC, Swartz MA (2006) Autologous morphogen gradients by subtle interstitial flow and matrix interactions. Biophys J 91:113–121
Friedman A, Lolas G (2005) Analysis of a mathematical model of tumor lymphangiogenesis. Math Models Methods Appl Sci 15(1):95–107
Galie P, Spilker RL (2009) A two-dimensional computational model of lymph transport across primary lymphatic valves. J Biomech Eng 131(11):111,004
Goldman J, Rutkowski JM, Shields JD, Pasquier MC, Cui Y, Schmokel HG, Willey S, Hicklin DJ, Pytowski B, Swartz MA (2007) Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J 21(4):1003–1012
Goodhill GJ (1997) Diffusion in axon guidance. Eur J Neurosci 9(7):1414–1421
Gosiewska A, Yi C, Blanc-Brude O, Geesin JC (1999) Characterization of a macrophage-based system for studying the activation of latent TGF-\(\beta \). Methods Cell Sci 21:47–56
Grainger DJ, Wakefield L, Bethell HW, Farndale RW, Metcalfe JC (1995) Release and activation of platelet latent TGF-beta in blood clots during dissolution with plasmin. Nat Med 1(9):932–937
Greenwood B (1973) The mitosis of sheep blood monocytes in tissue culture. Q J Exp Physiol 58:369–377
Haas TL, Duling BR (1997) Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles. Microvasc Res 53:113–120
Helm CL, Fleury ME, Zisch AH, Boschetti F, Swartz MA (2005) Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc Natl Acad Sci USA 102(44):15779–15784
Heppell C, Roose T, Richardson G (2015) A model for interstitial drainage through a sliding lymphatic valve. Bull Math Biol 77:1101–1131
Hormbrey E, Han C, Roberts A, McGrouther DA, Harris AL (2003) The relationship of human wound vascular endothelial growth factor (VEGF) after breast cancer surgery to circulating vegf and angiogenesis. Clin Cancer Res 9:4332–4339
Huggenberger R, Siddiqui SS, Brander D, Ullmann S, Zimmermann K, Antsiferova M, Werner S, Alitalo K, Detmar M (2011) An important role of lymphatic vessel activation in limiting acute inflammation. Blood 117(17):4667–4678
Hyytiäinen M, Penttinen C, Keski-Oja J (2004) Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci 41(3):233–264
Jeffcoate WJ, Harding KG (2003) Diabetic foot ulcers. Lancet 361(9368):1545–1551
Ji RC (2005) Characteristics of lymphatic endothelial cells in physiological and pathological conditions. Histol Histopathol 20:155–175
Kaminska B, Wesolowska A, Danilkiewicz M (2005) TGF beta signalling and its role in tumor pathogenesis. Acta Biochim Pol 52(2):329–337
Kaur H, Yung LY (2012) Probing high affinity sequences of DNA aptamer against VEGF165. PLoS ONE 7(2):e31,196
Khalil N, Corne S, Whitman C, Yacyshyn H (1996) Plasmin regulates the activation of cell-associated latent TGF-beta 1 secreted by rat alveolar macrophages after in vivo bleomycin injury. Am J Respir Cell Mol Biol 15(2):252–259
Khalil N, Whitman C, Zuo L, Danielpour D, Greenberg A (1993) Regulation of alveolar macrophage transforming growth factor-\(\beta \) secretion by corticosteroids in bleomycin-induced pulmonary inflammation in the rat. J Clin Invest 92:1812–1818
Kiriakidis S, Andreakos E, Monaco C, Foxwell B, Feldmann M, Paleolog E (2003) VEGF expression in human macrophages is NF-\(\kappa \)B-dependent: studies using adenoviruses expressing the endogenous NF-\(\kappa \)B inhibitor I\(\kappa \)B\(\alpha \) and a kinase-defective form of the I\(\kappa \)B kinase 2. J Cell Sci 116(4):665–674
Kleinheinz J, Jung S, Wermker K, Fischer C, Joos U (2010) Release kinetics of VEGF\(_{165}\) from a collagen matrix and structural matrix changes in a circulation model. Head Face Med 6:17. doi:10.1186/1746-160X-6-17
Krombach F, Munzing S, Allmeling AM, Gerlach JT, Behr J, Dorger M (1997) Cell size of alveolar macrophages: an interspecies comparison. Environ Health Perspect 105(Suppl 5):1261–1263
Lee S, Hwang HJ, Kim Y (2014) Modeling the role of TGF-\(\beta \) in regulation of the Th17 phenotype in the LPS-driven immune system. Bull Math Biol 76(5):1045–1080
Levine HA, Pamuk S, Sleeman BD, Nilsen-Hamilton M (2001) Mathematical modeling of capillary formation and development in tumor angiogenesis: penetration into the stroma. Bull Math Biol 63(5):801–863
Li Jeon N, Baskaran H, Dertinger SK, Whitesides GM, Van de Water L, Toner M (2002) Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nat Biotechnol 20(8):826–830
Lohela M, Bry M, Tammela T, Alitalo K (2009) VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol 21(2):154–165
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523(7560):337–341
Ludwig D, Aronson DG, Weinberger HF (1979) Spatial patterning of the spruce budworm. J Math Biol 8:217–258
Lunt SJ, Kalliomaki TM, Brown A, Yang VX, Milosevic M, Hill RP (2008) Interstitial fluid pressure, vascularity and metastasis in ectopic, orthotopic and spontaneous tumours. BMC Cancer 8:2
Mac Gabhann F, Popel AS (2004) Model of competitive binding of vascular endothelial growth factor and placental growth factor to VEGF receptors on endothelial cells. Am J Physiol Heart Circ Physiol 286:H153–H164
Macdonald AJ, Arkill KP, Tabor GR, McHale NG, Winlove CP (2008) Modeling flow in collecting lymphatic vessels: one-dimensional flow through a series of contractile elements. Am J Physiol Heart Circ Physiol 295(1):H305–313
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25(12):677–686
Mantzaris NV, Webb S, Othmer HG (2004) Mathematical modeling of tumor-induced angiogenesis. J Math Biol 49:111–187
Margaris KN, Black RA (2012) Modelling the lymphatic system: challenges and opportunities. J R Soc Interface 9(69):601–612
Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D’Amore PA (2007) Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol 70:1178–1191
May MTGT (1968) Galen on the usefulness of the parts of the body. Part II. Cornell University Press, Ithaca
Mendoza E, Schmid-Schönbein GW (2003) A model for mechanics of primary lymphatic valves. J Biomech Eng 125:407–414
Miura T, Tanaka R (2009) In vitro vasculogenesis models revisited—measurement of VEGF diffusion in matrigel. Math Model Nat Phenom 4(4):118–130
Monstrey S, Hoeksema H, Verbelen J, Pirayesh A, Blondeel P (2008) Assessment of burn depth and burn wound healing potential. Burns 34:761–769
Müller G, Behrens J, Nussbaumer U, Böhlen P, Birchmeier W (1987) Inhibitory action of transforming growth factor \(\beta \) on endothelial cells. PNAS 84:5600–5604
Murphy KE, Hall CL, Maini PK, McCue SW, McElwain DLS (2012) A fibrocontractive mechanochemical model of dermal wound closure incorporating realistic growth factor kinetics. Bull Math Biol 74(5):1143–1170
Nguyen VPKH, Chen SH, Trinh J, Kim H, Coomber BL, Dumont DJ (2007) Differential response of lymphatic, venous and arterial endothelial cells to angiopoietin-1 and angiopoietin-2. BMC Cell Biol 8:10
Norrmen C, Tammela T, Petrova TV, Alitalo K (2011) Biological basis of therapeutic lymphangiogenesis. Circulation 123(12):1335–1351
Nunes I, Shapiro RL, Rifkin DB (1995) Characterization of latent TGF-\(\beta \) activation by murine peritoneal macrophages. J Immunol 155:1450–1459
Oi M, Yamamoto T, Nishioka K (2004) Increased expression of TGF-\(\beta \)1 in the sclerotic skin in bleomycin-‘susceptible’ mouse strains. J Med Dent Sci 51:7–17
Oliver G, Detmar M (2002) The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev 16(7):773–783
Papaioannou AI, Zakynthinos E, Kostikas K, Kiropoulos T, Koutsokera A, Ziogas A, Koutroumpas A, Sakkas L, Gourgoulianis KI, Daniil ZD (2009) Serum VEGF levels are related to the presence of pulmonary arterial hypertension in systemic sclerosis. BMC Pulm Med 9:18
Pierce GF (2001) Inflammation in nonhealing diabetic wounds: the space–time continuum does matter. Am J Pathol 159(2):399–403
Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Jackson D, Skobe M (2002) Molecular characterization of lymphatic endothelial cells. PNAS 99(25):16069–16074
Posnett J, Franks PJ (2008) The burden of chronic wounds in the UK. Nurs Times 104(3):44–45
Reddy NP, Patel K (1995) A mathematical model of flow through the terminal lymphatics. Med Eng Phys 17(2):134–140
Rofstad EK, Galappathi K, Mathiesen BS (2014) Tumor interstitial fluid pressure—a link between tumor hypoxia, microvascular density, and lymph node metastasis. Neoplasia 16(7):586–594
Roose T, Fowler AC (2008) Network development in biological gels: role in lymphatic vessel development. Bull Math Biol 70(6):1772–1789
Rutkowski JM, Boardman KC, Swartz MA (2006) Characterization of lymphangiogenesis in a model of adult skin regeneration. Am J Physiol Heart Circ Physiol 291:H1402–H1410
Rutkowski JM, Swartz MA (2007) A driving force for change: interstitial flow as a morphoregulator. Trends Cell Biol 17(1):44–50
Saaristo A, Tammela T, Farkkila A, Karkkainen M, Suominen E, Yla-Herttuala S, Alitalo K (2006) Vascular endothelial growth factor-C accelerates diabetic wound healing. Am J Pathol 169:1080–1087
Schugart RC, Friedman A, Zhao R, Sen CK (2008) Wound angiogenesis as a function of tissue oxygen tension: a mathematical model. PNAS 105(7):2628–2633
Scianna M, Bell CG, Preziosi L (2013) A review of mathematical models for the formation of vascular networks. J Theor Biol 333:174–209
Sheikh AY, Gibson JJ, Rollins MD, Hopf HW, Hussain Z, Hunt TK (2000) Effect of hyperoxia on vascular endothelial growth factor levels in wound model. Arch Surg 135:1293–1297
Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA (2011) Latent TGF-\(\beta \) structure and activation. Nature 474:343–351
Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA (2007) Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine ccr7 signaling. Cancer Cell 11(6):526–538
Simonsen TG, Gaustad JV, Leinaas MN, Rofstad EK (2012) High interstitial fluid pressure is associated with tumor-line specific vascular abnormalities in human melanoma xenografts. PLoS ONE 7(6):e40,006
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341(10):738–746
Stadelmann WK, Digenis AG, Tobin GR (1998) Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg 176(2A Suppl):26S–38S
Stéphanou A, McDougall SR, Anderson ARA, Chaplain MAJ (2005) Mathematical modelling of flow in 2D and 3D vascular networks: applications to anti-angiogenic and chemotherapeutic drug strategies. Math Comput Model 41(10):1137–1156
Sutton AB, Canfield AE, Schor SL, Grant ME, Schor AM (1991) The response of endothelial cells to TGF\(\beta \)-1 is dependent upon cell shape, proliferative state and the nature of the substratum. J Cell Sci 99:777–787
Sweat RS, Stapor PC, Murfee WL (2012) Relationships between lymphangiogenesis and angiogenesis during inflammation in rat mesentery microvascular networks. Lymphat Res Biol 10(4):198–207
Swift ME, Burns AL, Gray KL, DiPietro LA (2001) Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol 117(5):1027–1035
Tammela T, Alitalo K (2010) Lymphangiogenesis: molecular mechanisms and future promise. Cell 140:460–476
Taylor AW (2009) Review of the activation of TGF-\(\beta \) in immunity. J Leukoc Biol 85:29–33
Tranquillo RT, Zigmond SH, Lauffenburger DA (1988) Measurement of the chemotaxis coefficient for human neutrophils in the under-agarose migration assay. Cell Motil Cytoskelet 11(1):1–15
van den Berg BM, Vink H, Spaan JAE (2003) The endothelial glycocalyx protects against myocardial edema. Circ Res 92:592–594
Velázquez JJL (2004a) Point dynamics in a singular limit of the Keller–Segel model 1: motion of the concentration regions. SIAM J Appl Math 64(4):1198–1223
Velázquez JJL (2004b) Point dynamics in a singular limit of the Keller–Segel model 2: formation of the concentration regions. SIAM J Appl Math 64(4):1224–1248
von Staden H (1989) Herophilus [and] the art of medicine in early alexandria. Cambridge University Press, Cambridge
Vowden P (2011) Hard-to-heal wounds Made Easy. Wounds Int 2(4). http://www.woundsinternational.com
Wahl SM, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB, Sporn MB (1987) Transforming growth factor type \(\beta \) induces monocyte chemotaxis and growth factor production. PNAS 84:5788–5792
Wakefield LM, Smith DM, Flanders KC, Sporn MB (1988) Latent transforming growth factor-beta from human platelets. A high molecular weight complex containing precursor sequences. J Biol Chem 263(16):7646–7654
Waugh HV, Sherratt JA (2006) Macrophage dynamics in diabetic wound healing. Bull Math Biol 68:197–207
Weber-Matthiesen K, Sterry W (1990) Organization of the monocyte/macrophage system of normal human skin. J Invest Dermatol 95:83–89
Whitehurst B, Eversgerd C, Flister M, Bivens CM, Pickett B, Zawieja DC, Ran S (2006) Molecular profile and proliferative responses of rat lymphatic endothelial cells in culture. Lymph Res Biol 4(3):119–142
Withington ETT (1984) Hippocrates on joints, vol 3. Harvard University Press, Cambridge
Witte MH, Bernas MJ, Martin CP, Witte CL (2001) Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc Res Tech 55:122–145
Yang JP, Liu HJ, Cheng SM, Wang ZL, Cheng X, Yu HX, Liu XF (2009) Direct transport of VEGF from the nasal cavity to brain. Neurosci Lett 449(2):108–111
Yang L, Qiu CX, Ludlow A, Ferguson MWJ, Brunner G (1999) Active transforming growth factor-\(\beta \) in wound repair—determination using a new assay. Am J Pathol 154(1):105–111
Zachary I, Gliki G (2001) Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res 49:568–581
Zheng Y, Watanabe M, Kuraishi T, Hattori S, Kai C, Shibuya M (2007) Chimeric VEGF-ENZ7/PlGF specifically binding to VEGFR-2 accelerates skin wound healing via enhancement of neovascularization. Arterioscler Thromb Vasc Biol 27(3):503–511
Zhuang JC, Wogan GN (1997) Growth and viability of macrophages continuously stimulated to produce nitric oxide. PNAS 94:11875–11880
Zimny S, Schatz H, Pfohl M (2002) Determinants and estimation of healing times in diabetic foot ulcers. J Diabetes Complic 16(5):327–332
Acknowledgments
A.B. was funded in part by a Maxwell Institute Scholarship from Heriot-Watt University. K.J.P. acknowledges partial support from BBSRC Grant BB/J015940/1.
Author information
Authors and Affiliations
Corresponding author
Appendix: Parameter Estimation
Appendix: Parameter Estimation
1.1 Sizes, Weights, Equilibria and Velocities
1.1.1 Domain Size
We consider a full-thickness wound of length \(\ell =5 \text{ mm }\), inspired by Zheng et al. (2007). For the surrounding skin, we consider a (small) variable width \(\varepsilon \). Thus, we have a domain of length \(5 \text{ mm } + 2\varepsilon \). In all the simulations reported in the present paper, \(\varepsilon =1\); the nature of the observations does not change if a different value of \(\varepsilon \) is chosen (simulations not shown).
1.1.2 TGF-\(\beta \) Molecular Weight and Equilibrium \(T^\mathrm{eq}\)
We take TGF-\(\beta \) molecular weight to be approximately 25 kDa (Boulton et al. 1997; Wakefield et al. 1988, active/mature isoform). The equilibrium value of active TGF-\(\beta \) is about 30 pg/mm\(^3\) (Yang et al. 1999, Figure 2).
1.1.3 Macrophage Volume and Equilibrium \(M^\mathrm{eq}\)
A human alveolar macrophage has a volume \(V_{M\Phi }\) of approximately \(5000 \mu \text{ m }^3 = 5\times 10^{-6} \text{ mm }^3\) (Krombach et al. 1997). The macrophage steady state can be estimated from Weber-Matthiesen and Sterry (1990, Figure 1), which plots typical macrophage density in the skin. This shows that there is an average of about 15 macrophages per 0.1mm\(^2\) field. Assuming a visual depth of 80 \(\mu \)m, the macrophage density becomes 15 cells/(0.1mm\(^2\times 0.08\)mm) \(=\) 1875 cells/mm\(^3\).
1.1.4 VEGF Molecular Weight and Equilibrium \(V^\mathrm{eq}\)
VEGF molecular weight is taken to be 38 kDa (Kaur and Yung 2012; Yang et al. 2009, VEGF-165). The VEGF equilibrium concentration is estimated to be 0.5 pg/mm\(^3\) from Hormbrey et al. (2003, Figure 1) and Papaioannou et al. (2009, Figure 2).
1.1.5 Normal Capillary Density \(C^\mathrm{eq}\)
In Rutkowski et al. (2006), we find that “it was not until day 60, when functional and continuous lymphatic capillaries appeared normal” and “at day 60 the regenerated region had a complete lymphatic vasculature, the morphology of which appeared similar to that of native vessels”. Hence, we assume that a capillary network that can be considered “final” appears at day 60, and we take \(C^\mathrm{eq}\) to be the number of LECs present at this time. In Rutkowski et al. (2006, Figure 2E), we see that at that time there are about 80 cells. This value corresponds to a 12 \(\mu \)m thin section. In addition, from Rutkowski et al. (2006, Figure 2D) we can calculate the observed wound area, which is about \(5.6\times 10^5 \, \mu \text{ m }^2\). In this way, we get a volume of 0.0067 mm\(^3\) with 80 cells, which corresponds to \(C^\mathrm{eq}=1.2\times 10^4\) cells/mm\(^3\).
1.1.6 Maximum Capillary Density \(C_{\max }\)
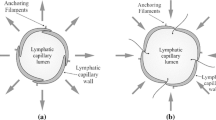
First of all, we want to convert 1 capillary section into a cell number. For this purpose, we assume EC cross-sectional dimensions to be those reported in Haas and Duling (1997), namely \(10\,\mu \text{ m }\times 100\,\mu \text{ m }\). We then assume that LECs lie “longitudinally” along the capillaries, and therefore, only the short dimension contributes to cover or “wrap” the circumference of the capillary. Considering a capillary diameter of 55 \(\mu \)m as in Fischer et al. (1996), we have that each lymphatic capillary section is made of approximately 20 LECs (taking into account some overlapping). Then, from van der Berg et al. (2003) we know that EC thickness is approximately 0.5 \(\mu \)m. Thus, a capillary section is a circle of about \(55+2\times 0.5 \, \mu \)m diameter, corresponding, as described above, to 20 cells.
If we imagine stacking 1 mm\(^3\) with capillaries of this size, we see that we can pile on \(1 \text{ mm }/ 56\,\mu \text{ m } \approx 18\) layers of capillaries. Then, considering an EC length of 100 \(\mu \)m as in Haas and Duling (1997), we have that 1 mm\(^3\) fits at most a number of capillaries equivalent to the following amount of ECs:
1.1.7 Lymph Velocity
Fischer et al. (1996) suggests that the high lymph flow value (0.51mm/s) is due to high pressure following die injection. This suggests that a lower value (9.7 microns/s) might be considered as typical, in agreement with Fischer et al. (1997). In both papers, the normal lymph velocity seems to be around 10 microns/s.
We thus assume lymph velocity to be \(v_\mathrm{lymph}\) \(=\) 10 micron/s \(=\) 864 mm/day (from Fischer et al. 1996, 1997).
1.1.8 Interstitial Flow Velocity
First of all, we note that in Rutkowski and Swartz (2007) interstitial flow in the skin is calculated to be around 10 microns/s. [Note that Helm et al. (2005) is relevant for this aspect of our modelling, although it is less important for the estimation of parameters; in this reference, the synergy between interstitial flow and VEGF gradient is discussed.] Therefore, we will consider the interstitial flow to be also \(v_{IF}\) \(=\) 10 microns/s \(=\) 864 mm/day (from Rutkowski and Swartz 2007).
1.2 Re-calculation of \(s_M\) and \(k_1\)
\(s_M\) here is calculated in the same way as in Bianchi et al. (2015), but using our amended model equations presented here. For \(k_1\), we point out that in Bianchi et al. (2015) this parameter was appearing in the logistic part of the M-equation: \({{d M}/{dt} = r_2 M - {r_2}/{k_1}\cdot M^2}\). In the PDE systems, we do not include such terms because only a minor fraction of macrophages undergo mitosis (Greenwood 1973). However, death due to overcrowding is present in both models; comparing these terms, we see that our “new” \(k_1\) corresponds to the “old” \(k_1 / r_2\).
1.3 Diffusion Coefficients
1.3.1 VEGF Diffusion Coefficient \(D_V\)
In Miura and Tanaka (2009), the authors observe that “in general, the diffusion coefficient of protein molecules in liquid is of the order of \(10^6\,\mu \text{ m }^2/\text{ h }=24\,\text{ mm }^2/\text{ day }\). This intuitively means that a molecule moves 10 \(\mu \)m/s. To generate a gradient over the order of 100 \(\mu \)m, the timescale of protein decay should be around 10 s. In this specific case, the protein decay time is about 1–10 h. Therefore, the observed diffusion coefficient is too large and we need some mechanism to slow down the diffusion” (where “this specific case” means that of VEGF).
In Miura and Tanaka (2009) the VEGF diffusion coefficient is estimated in three different ways: by a theoretical model (\(0.24 \text{ mm }^2/\text{ day }\)), and by two different empirical techniques (\(24 \text{ mm }^2/\text{ day }\)). The authors then suggest a diffusion coefficient of the order of \(10^6\,\mu \text{ m }^2/\text{ h }=24 \text{ mm }^2/\text{ day }\). However, they also used the same technique to determine the diffusion coefficient at the cell surface; this time the diffusion coefficient is estimated to be approximately \(10^4\,\mu \text{ m }^2/\text{ h }=0.24\,\text{ mm }^2/\text{ day }\). Keeping in mind all these considerations, for the model we take the intermediate value \(D_V = 2.4\,\text{ mm }^2/\text{ day }\).
1.3.2 TGF-\(\beta \) Diffusion Coefficient \(D_T\)
In Lee et al. (2014) the authors estimate a TGF-\(\beta \) diffusion coefficient of 0.36 mm\(^2\)/h \(=\) 8.64 mm\(^2\)/day from Brown (1999), Goodhill (1997). In Murphy et al. (2012), the authors estimate a TGF-\(\beta \) diffusion coefficient of 2.54 mm\(^2\)/day using the Stokes–Einstein Formula.
We checked their consistency with the estimate for \(D_V\) above. The Stokes–Einstein equation of these calculated values assumes spherical particles of radius r to have diffusion coefficient \(D\sim {1}/{r}\); since the molecular weight w of a particle is proportional to its volume, we have that \(D\sim {1}/{\root 3 \of {w}}\) and thus \(D_T \approx 2.76\).
1.3.3 Macrophage Random Motility \(\mu _M\)
In Farrell et al. (1990), we find “Population random motility was characterised by the random motility coefficient, \(\mu \), which was mathematically equivalent to a diffusion coefficient. \(\mu \) varied little over a range of C5a [a protein] concentrations with a minimum of \(0.86 \times 10^{-8} \text{ cm }^2/\text{ sec }\) in \(1\times 10^{-7}\) M C5a to a maximum of \(1.9\times 10^{-8} \text{ cm }^2/\text{ sec }\) in \(1\times 10^{-11}\) M C5a”. We thus take \(\mu _M\) to be the average of these two values, that is \(\mu _M = 1.38\times 10^{-8}\text{ cm }^2/\text{ s }\approx 0.12 \text{ mm }^2/\text{ day }\).
1.4 Advection Parameters \(\lambda _1\) and \(\lambda _2\)
We will take \(\lambda _2^\mathrm{chem}\) to be equal to \(v_{IF}\) calculated in “Appendix of Sizes, Weights, Equilibria and Velocities”; thus, \(\lambda _2^\mathrm{chem}\) = 864 mm/day. For \(\lambda _1^\mathrm{chem}\), it is more complicated, but we would say that if \(C_\mathrm{op}\) reaches the maximum possible value \(C_{\max }\) calculated in “Appendix of Maximum Capillary Density \(C_{\max }\)”, then \(\lambda _1^\mathrm{chem}\cdot C_\mathrm{op} = v_\mathrm{lymph}\), which was calculated in “Appendix of Sizes, Weights, Equilibria and Velocities”. That is, we assume that if the skin is “packed” with open capillaries, then the resulting flow will be the same as the usual lymph flow in the skin lymphatics). Hence, \(\lambda _1^\mathrm{chem} = v_\mathrm{lymph}/C_{\max } = 0.0135 \text{ mm } \text{ day }^{-1}\text{ cell }^{-1}\). For cells, we assume smaller values due the higher friction that cells encounter in the tissue. In the absence of relevant empirical data, we take \({\lambda _1^\mathrm{cell}={1}/{10}\cdot \lambda _1^\mathrm{chem}}\) and \({\lambda _2^\mathrm{cell}={1}/{10}\cdot \lambda _2^\mathrm{chem}}\).
1.5 Rate at Which TGF-\(\beta \) is Internalised by Macrophages \(\gamma _1\)
At equilibrium, \(C=C^\mathrm{eq}\) and thus \(p(C)=0\). Therefore, the equation for T at equilibrium becomes
which leads to
1.6 Chemotaxis Parameters
1.6.1 Macrophage Chemotactic Sensitivity Towards TGF-\(\beta \) \(\chi _1\)
In Li Jeon et al. (2002, Table 1), the chemotaxis coefficients of neutrophils for different gradients of interleukin-8 are listed (ranging from \(0.6\times 10^{-7}\) to \(12\times 10^{-7}\) mm\(^2\cdot \)mL\(\cdot \)ng\(^{-1}\cdot \)s\(^{-1}\)). We take the intermediate value \(\chi _1 = 5\times 10^{-7}\text{ mm }^2\text{ mL } \text{ ng }^{-1}\text{ s }^{-1}\approx 4\times 10^{-2}\text{ mm }^2(\text{ pg/mm }^3)^{-1}\text{ day }^{-1}\). To compare this value with one from another source, we consider Tranquillo et al. (1988, Figure 8): although the chemotaxis coefficient is shown to depend on the attractant concentration, an average value is \(\chi = 150 \text{ cm }^2\text{ sec }^{-1}\text{ M }^{-1}\approx 5.18\times 10^{-2}\text{ mm }^2(\text{ pg/mm }^3)^{-1}\text{ day }^{-1}\) (using the TGF-\(\beta \) molecular weight found in “Appendix of TGF-\(\beta \) Molecular Weight and Equilibrium \(T^\mathrm{eq}\)”). This result is encouraging because it is of the same order of magnitude as the previous estimate.
1.6.2 LEC Chemotactic Sensitivity Towards VEGF \(\chi _2\)
In Barkefors et al. (2008), a quantification is made of the effects of FGF2 and VEGF165 on HUVEC and HUAEC chemotaxis. In Barkefors et al. (2008, Figure 6A), it is reported that the total distance migrated per HUVEC in response to a 50 ng/mL gradient of VEGFA165 was about 150 \(\mu \)m. Considering that the analysed area of the cell migration chamber was 800 \(\mu \)m long and that the experiment lasted 200 minutes, we can estimate the endothelial cell velocity to be 150/200 = 0.75 \(\mu \)m/min = 1.08 mm/day and the VEGF gradient to be 50 ng/mL / 800 \(\mu \)m = 62.50 (pg/mm\(^3\))/mm. Now, the flux \(\mathcal {J}\) in our equation is given by \(\mathcal {J} = \chi _2 L \frac{\partial V}{\partial x}\); however, \(\mathcal {J}\) can also be seen as the product of the mass density and the velocity of the flowing mass (Douglas et al. 2005). Therefore, with L being our mass density, we have
and then we can use the previous calculations to estimate
In order to have realistic cell movement dynamics, \(\chi _2\) is taken to be 10 times bigger. This can be justified by the fact that the aforementioned data refer to HUVECs, and LECs might be faster than these cell types. A more suitable data set for this parameter would be very useful to better inform this estimate, but we are not aware of such data. Also, chemical gradients created in vitro are usually different between those observed in vivo and they are known to highly affect cell velocity.
1.6.3 Density Dependence of the Macrophage Chemotactic Sensitivity \(\omega \)
The cell density dependence of the macrophage velocity is given by the factor \(1/(1+\omega M)\). This velocity is maximal when M is close to zero, and we assume that it is halved when M reaches its carrying capacity \(k_1^{old}\) (that is, the parameter \(k_1\) in Bianchi et al. (2015)). We therefore take \(\omega \) to be the inverse of the macrophage-carrying capacity \(k_1^{old}\).
1.7 Macrophage Inflow \(\phi _1\)
We expect \(\phi _1\) to be proportional to the lymph flow (estimated in “Appendix of Sizes, Weights, Equilibria and Velocities” as \(v_\mathrm{lymph} = 864 \text{ mm } \text{ day }^{-1}\)) and macrophage presence in the lymph. In the same source Fischer et al. (1996) that we used to estimate \(v_\mathrm{lymph}\), it is reported that the mean capillary diameter is 55 \(\mu \)m. Thus, about \(2.05 \text{ mm }^3\) of lymph pass through a capillary bi-dimensional section in 1 day.
In Cao et al. (2005), we find that a mouse leucocyte count in the blood is approximately 3 to \(8\times 10^6\) cells/mL and that of these about \(2\times 10^6\) are macrophages coming from the lymph nodes; so we have a macrophage density of \(2\times 10^3 \text{ cells/mm }^3\) in the lymph. Therefore, each day about \(2.05 \text{ mm }^3 \times 2\times 10^3 \text{ cells/mm }^3 = 4.11\times 10^3\) macrophages pass in one capillary. Converting capillaries into cell density as was done in “Appendix of Maximum Capillary Density \(C_{\max }\)”, we have an influx equal to \(\frac{4.11}{20}\times 10^3\text{ day }^{-1}=0.205\times 10^3 \text{ day }^{-1}\). However, the macrophage density reported in Cao et al. (2005) refers to blood; we assume that this quantity in lymph (especially during inflammation) will be about 10 times bigger. Therefore, we will take \(\phi _1 = 2.05\times 10^3 \text{ day }^{-1}\).
Rights and permissions
About this article
Cite this article
Bianchi, A., Painter, K.J. & Sherratt, J.A. Spatio-temporal Models of Lymphangiogenesis in Wound Healing. Bull Math Biol 78, 1904–1941 (2016). https://doi.org/10.1007/s11538-016-0205-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-016-0205-x