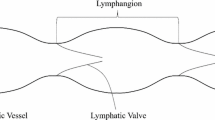
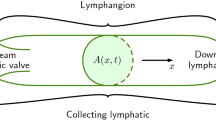
Abstract
This study investigates fluid flow and elastic deformation in tissues that are drained by the primary lymphatic system. A model is formulated based on the Rossi hypothesis that states that the primary lymphatic valves, which are formed by overlapping endothelial cells around the circumferential lining of lymphatic capillaries, open in response to swelling of the surrounding tissue. Tissue deformation and interstitial fluid flow through the tissue are treated using the Biot equations of poroelasticity and, the fluid flux (into the interstitium) across the walls of the blood capillaries, is assumed to be linearly related to the pressure difference across the walls via a constant of proportionality (the vascular permeability). The resulting model is solved in a periodic domain containing one blood capillary and one lymphatic capillary starting from a configuration in which the tissue is undeformed. On imposition of a constant pressure difference between blood and lymphatic capillaries, the solutions are found to settle to a steady state. Given that the magnitude of pressure fluctuations in the lymphatic system is much smaller than this pressure difference between blood and lymph, it is postulated that the resulting steady- state solution gives a good representation of the state of the tissue under physiological conditions. The effects of changes to the Young’s modulus of the tissue, the blood-lymphatic pressure difference, vascular permeability and valve dimensions on the steady state are investigated and discussed in terms of their effects on oedema in the context of age- and pregnancy-related changes to the body.












Similar content being viewed by others
Notes
See Table 2 for definitions of all the dimensionless parameter groupings.
References
Anim-Nyame N, Gamble J, Sooranna S, Johnson M, Steer P (2003) Microvascular permeability is related to circulating levels of tumor necrosis factor—\(\alpha \) in pre-eclampsia. Cardiovasc Res 58:162–169
Basser PJ (1992) Interstitial pressure, volume, and flow during infusion into brain tissue. Microvasc Res 44:143–165
Bates D (2010) Vascular endothelial growth factors and vascular permeability. Cardiovasc Res 87:202–271
Baxter L, Jain R (1990) Transport of fluid and macromolecules in tumors. II. Role of heterogeneous perfusion and lymphatics. Microvasc Res 40:246–263
Biot MA (1956) General solutions of the equations of elasticity and consolidation for a porous material. J Appl Mech 78:91–96
Bowen R (1980) Incompressible porous media models by use of the theory of mixtures. Int J Eng Sci 18:1129–1148
Burton AC (1951) On the physical equilibrium of small blood vessels. Am Physiol Soc 164:319–329
Casley-Smith J (1976) The functioning and interrelationships of blood capillaries and lymphatics. Cell Mol Life Sci 32:1–12
Cho S, Atwood JE (2002) Peripheral edema. Am J Med 113:580–586
Danias P, Tritos N, Stuber M, Botnar R, Kissinger K, Manning W (2003) Comparison of aortic elasticity determined by cardiovascular magnetic resonance imaging in obese versus lean adults. Am J Cardiol 91:195–199
Davis M, Lane M, Davis A, Durtschi D, Zawieja D, Muthuchamy M, Gasher A (2008) Modulation of lymphatic muscle contractility by the neuropeptide substance P. Am J Physiol 295:587–597
Ding M, Dalstra M, Danielsen C, Tabel J, Hvid I, Linde F (1997) Age variations in the properties of human tibial trabecular bone. J Bone Joint Surg 79:995–1002
Dixon J (2010) Lymphatic lipid transport: sewer or subway. Trends Endocrinol Metab 21:480–487
Dixon JB, Greiner ST, Goshev AA, Cote GL, Moore JE Jr, Zawieja DC (2006) Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 13:597–610
Eisenhoffer J, Johnston M (1995) Importance of valves and lymphangion contractions in determining pressure gradients in isolated lymphatics exposed to elevations in outflow pressure. Microvasc Res 49:97–110
Flyvbjerg A, Dagnaes-Hansen F, De Vriese A, Schrijvers B, Tilton R, Rasch R (2002) Amelioration of long term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes 51:3090–3094
Friedman C, Danforth D, Herbosa-Encarnacion C, Arbogast L, Alak B, Seifer D (1997) Follicular fluid vascular endothelial growth factor concentrations are elevated in women of advanced reproductive age undergoing ovulation induction. Fertil Steril 68:607–612
Galie P, Spilker RL (2009) A two-dimensional computational model of lymph transport across primary lymphatic valves. J Biomech Eng 131:1297–1307
Geleff SB, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D (1999) Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries. Am J Pathol 154:385–394
Heppell CW, Richardson G, Roose T (2012) A model for fluid drainage by the lymphatic system. Bull Math Biol 75:49–81
Hitchcock T, Niklason L (2008) Lymphatic tissue engineering progress and prospects. Ann N Y Acad Sci 1131:44–49
Holmes M (1986) Finite deformation of soft tissue: analysis of a mixture model in uni-axial compression. J Biomech Eng 108:372–381
Holmes M (2009) Appl Solid Mech. Cambridge University Press, Cambridge
Ikomi F, Hunt J, Hanna G, Schmid-Schönbein GW (1996) Interstitial fluid, plasma protein, colloid, and leukocyte uptake into initial lymphatics. J Appl Physiol 81:2060–2067
Jain RK, Tong RT, Munn LL (2007) Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: Insights from a mathematical model. Am Assoc Cancer Res 67:2729–2735
Kaczmarek M, Subramaniam R, Neff S (1997) The hydromechanics of hydrocephalus: steady–state solutions for cylindrical geometry. Bull Math Biol 59:295–323
Kärkkäinen H, Saarelainen H, Valtonen P, Laitinen T, Raitakari O, Juonala M, Kähönen M, Hutri-Kähönen N, Heinonen S, Laitinen T (2014) Carotid artery elasticity decreases during pregnancy—the cardiovascular risk in young finns study. BMC Pregnancy Child Birth 14:98–108
Keaveny T, Hayes W (1993) A 20-year perspective on the mechanical properties of trabecular bone. J Biomech Eng 115:534–542
Kvanter A, Algvere P, Berglin L, Seregard S (1996) Subfoveal degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci 37:1929–1934
Leak LV (1970) Electron microscopic observations on lymphatic capillaries and the structural components of the connective tissue-lymph interface. Microvasc Res 2:361–391
Leak LV (1971) Studies on the permeability of lymphatic capillaries. J Cell Biol 50:300–323
Leak LV, Burke JF (1968) Ultrastructural studies on the lymphatic anchoring filaments. J Cell Biol 36:129–149
Lee JS (2004) Tissue fluid pressure, lymph pressure, and fluid transport in rat intestinal villi. Microvasc Res 31:170–183
Levick JR (1987) Flow through interstitium and other fibrous matrices. J Exp Physiol 72:409–439
Macedo M, Luminoso D, Savvidou M, McEniery C, Nicolaides K (2008) Pregnancy, hypertension, and preeclampsia. Hypertension 51:1047–1051
Margaris KN, Black RA (2012) Modelling the lymphatic system: challenges and opportunities. J R Soc Interface 9:601–612
Mattei A, Fuechsel F, Dhar N, Warnike S, Thalmann G, Krause T, Studer U (2008) The template of the primary lymphatic landing sites of the prostate should be revisited: results of a multimodality mapping study. Eur Urol 53:118–125
Mendoza E, Schmid-Schönbein GW (2003) A model for mechanics of primary lymphatic valves. J Biomech Eng 125:407–414
Moriondo A, Boschetti F, Bianchin F, Lattanzio S, Marcozzi C, Negrini D (2010) Tissue contribution to the mechanical features of diaphragmatic initial lymphatics. J Physiol 588:3957–3969
Nipper M, Dixon J (2011) Engineering the lymphatic system. Cardiovasc Eng Technol 2:296–308
Pusenjak J, Miklavcic D (2000) Modeling of interstitial fluid pressure in solid tumor. Simul Pract Theory 8:17–24
Reddy NP, Patel K (1994) A mathematical model of flow through the terminal lymphatics. Med Eng Phys 17:134–140
Reddy ST, Berk DA, Jain RK, Swartz MA (2006) A sensitive in vivo model for quantifying interstitial convective transport of injected macromolecules and nanoparticles. J Appl Phys 101:1162–1169
Roose T, Fowler A (2008) Network development in biological gels: roles in lymphatic vessel development. Bull Math Biol 70:1772–1789
Roose T, Swartz M (2012) Multiscale modeling of lymphatic drainage from tissues using homogenization theory. J Biomech 45:107–115
Roose T, Tabor G (2013) Multiscale modelling of lymphatic drainage. In: Gefen A (ed) Studies in mechanobiology, tissue engineering and biomaterials, vol 14. Springer, Berlin, Heidelberg, pp 149–176
Rossi A, Weber E, Sacchi G, Maestrini D, Di Cintio F, Gerli R (2007) Mechanotransduction in lymphatic endothelial cells. Lymphology 40:102–113
Sarntinoranont M, Rooney F, Ferrari M (2003) Interstitial stress and fluid pressure within a growing tumor. Biomed Eng Soc 31:327–335
Sauter B, Foedinger D, Sterniczky B, Wolff K, Rappersberger K (1998) Immunoelectron microscopic characterization of human dermal lymphatic microvascular endothelial cells: \(\cdots \). J Histochem Cytochem 46:165–176
Sherratt M (2009) Tissue elasticity and the ageing elastic fibre. Age 31:305–325
Staub N (1978) Pulmonary edema due to increased microvascular permeability to fluid and protein. Circul Res 43:143–151
Swartz MA (2001) The physiology of the lymphatic system. Adv Drug Deliv Rev 50:3–20
Swartz MA, Boardman KC (2002) The role of interstitial stress in lymphatic function and lymphangiogenesis. Ann N Y Acad Sci 979:197–210
Swartz MA, Kaipainen A, Netti PA, Brekken C, Boucher Y, Grodzinsky AJ, Jain RK (1999) Mechanics of interstitial-lymphatic fluid transport: theoretical foundation and experimental validation. J Biomech 32:1297–1307
Unthank J, Bohlen H (1988) Lymphatic pathways and role of valves in lymph propulsion from small intestine. Am J Physiol 254:389–398
Wilaman R, Mackey R, Bostom A, Thompson T, Sulton-Tyrrell K (2003) Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension 42:408–473
Zweifach B, Prather J (1975) Micromanipulation of pressure in terminal lymphatics in the mesentery. Am J Physiol 228:1326–1335
Acknowledgments
C.W.H. was funded by EPSRC Ph.D. studentship. T.R. was funded by a Royal Society University Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Appendix: Numerical Solution of the Model Using Comsol
Appendix: Numerical Solution of the Model Using Comsol
Here we describe how we use the finite element package Comsol Multiphysics (CM) to solve the model formulated in Sect. 2. At its most basic, this comprises of comparing the poroelasticity equations defined in CM to Eqs. (7) and (11) and interpreting the boundary conditions (18)–(31) in a form appropriate for CM and then solving for the solid matrix deformations and the interstitial fluid flow.
1.1 The Poroelastic Equations
CM has a poroelastic module that can be used to find numerical solutions to Biot’s equations. This couples together a Darcy’s Law interface (for the flow) to a solid mechanics interface (for the mechanical deformations), which we describe below.
1.1.1 Darcy’s Law Interface
This is used to determine fluid motion through a porous medium. Darcy’s law states that the fluid flux (\(\mathbf Q ^{f}\)) is linearly related to the pressure gradient in the medium (\(\nabla P\)) via the relation
where \({\mu }\) is fluid viscosity and \(\kappa \) the permeability of the porous medium (for consistency with Sect. 2 \(K=\kappa /\upmu \)). This is solved together with the fluid continuity equation, which is provided by the CM Poroelasticity Interface
where for consistency with Sect. 2, we set the Biot-Willis coefficient \(\alpha _b=1\).
1.1.2 Solid Mechanics Interface
Following the small displacement assumption, the normal strain components and the shear strain components, \(\epsilon _{ij}\), are given from the deformations, \(u_{i}\), via
and, since we assume plane strain \({\varvec{u}}=(u_1(x_1,x_2), u_2(x_1,x_2),0)\), it follows that \(\epsilon _{13}=\epsilon _{23}=\epsilon _{33}=0\).
For a poroelastic material, the stress tensor (\(\sigma _{ij}\)) is related to the strain tensor by the standard linear constitutive relation \(\sigma _{ij}=D_{ijkl} \epsilon _{kl}-P \alpha _b \delta _{ij}\) where the term \(-P \alpha _b \delta _{ij}\) is added to account for the pore pressure (here \(D_{ijkl}\) is entered into the solid mechanics interface). For isotropic materials, this can be reduced [see e.g. Holmes (2009)] to the form
and \(E\) is Young’s modulus and \(\nu \) is the Poisson’s ratio. Under the assumptions of plane strain \(\sigma _{13}=\sigma _{31}=0\) and the force balance equations (that are entered into the structural mechanics module) are
The stresses playing a role in the force balance equation are related to the nonzero strain tensor components via
The governing equations are (49)–(51) and (53)–(55), with \(\alpha _b=1\), are identical to those in Sect. 2 (i.e. (7) and (11)).
1.1.3 Boundary Conditions
Here we convert our boundary conditions (on the sections of boundary illustrated in Fig. 3) to a form usable by CM.
The Lymphatic Capillary Wall \(\partial \Omega _{4}\)
The lymphatic capillary wall (\(\partial \Omega _{4}\)) is split into two parts, which are denoted by \(\partial \Omega _{4a}\) and \(\partial \Omega _{4b}\), see Fig. 3. Here the boundary \(\partial \Omega _{4a}\) corresponds to the open section of the lymphatic valve (through which interstitial fluid flows), while \(\partial \Omega _{4b}\) represents the endothelial cell wall (through which there is no flow). We assume that the open section of the lymphatic valve is located half way along the capillary boundary, as illustrated in Fig. 3.
We determine the positions of \(\partial \Omega _{4a}\) and \(\partial \Omega _{4b}\) by introducing two new variables
Here \(\theta _{s}\) and \(\theta _{f}\) are the angles from the horizontal to the start of \(\partial \Omega _{4a}\) and the end of \(\partial \Omega _{4a}\), respectively. Thus, \(\partial \Omega _{4a} = \{ \theta | \theta \in [\theta _s,\theta _f] \}\) and \(\partial \Omega _{4b} = \{ \theta | \theta \in [0,\theta _s) \} \cup \{ \theta | \theta \in (\theta _f, \pi /2) \}\), where \(\theta \) is the angle from the horizontal.
Using these angles in CM allows us to use a single flow boundary condition on \(\partial \Omega _4\) and still incorporate the valve mechanics. We determine \(C(t)\) by evaluating the boundary integral \(C(t)=\int _{\partial \Omega _{4}(t)} \hbox {d}S\). The assumption that the lymphatic valve is closed when \(C(t)<C_{\mathrm{crit}}\), and open when \(C(t)>C_{\mathrm{crit}}\) implies there is no flow into the lumen if \(C(t)<C_{\mathrm{crit}}\) and that fluid flows into the lumen only through \(\partial \Omega _{4a}\) when \(C(t)>C_{\mathrm{crit}}\). The boundary condition is implemented in CM by the command
This implies that
where \(P^{l}\) is the fluid pressure of the lymphatic lumen. It is thus equivalent to (18)–(19), the conditions derived in Sect. 2.
The Blood Capillary Wall, Boundary \(\partial \Omega _{1}\)
The blood capillary wall is permeable to fluid, but provides a resistance to it. We use the boundary condition Pervious Layer in CM to model the flow through this wall. This describes a mass flux through a semi-pervious layer connected to an external fluid source at given pressure (\(P^{b}\)) and is equivalent to
and so, where \({\kappa }/{\mu }=K\), is identical to (20).
Remaining Edges of the Periodic Tile, Boundaries \(\partial \Omega _{2}\), \(\partial \Omega _{3}\), \(\partial \Omega _{5}\) and \(\partial \Omega _{6}\)
The conditions (22), namely
are represented by the ‘No Flow’ feature in CM.
Solid Boundary Conditions
Since the periodic tile must remain square as it expands and contracts, its edges have to remain horizontal (\(\partial \Omega _{2}\) and \(\partial \Omega _{5}\)) and vertical (\(\partial \Omega _{3}\) and \(\partial \Omega _{6}\)). Without loss of generality we impose zero normal tissue displacement on boundaries \(\partial \Omega _{2}\) and \(\partial \Omega _{6}\), but allow them to deform freely in the tangential direction; the latter is equivalent to imposing zero tangential shear stress along the boundaries. These conditions are represented by the Roller condition in CM, that is
The edges \(\partial \Omega _{3}\) and \(\partial \Omega _{5}\) of the periodic tile deform freely in tangential direction (equivalent to tangential shear stress). However, both of these edges are displaced normally by a distance \(u_{c}(t)\), which we shall calculate. The Prescribed Displacement feature in CM imposes a condition in which the position of the boundary of the solid matrix is prescribed in one direction and free to deform in the other direction, and in our case is used to impose the conditions
where \(u_{c}(t)\) is calculated by
Here \(L(t)\) is the width of the periodic tile, which is a square, minus the radius of the lymphatic capillary.
The first integral term on the right-hand side of (67) cannot be calculated directly in CM because it is a time-dependent integral and the inbuilt time integrals in CM (timeint and timeavg) are only available during the results evaluation. In order to overcome this difficulty we introduce the new time-dependent variable, \(T_{1}(t)\), defined as the solution to
so that
It follows from (19) and (20) that
However, the tangential derivative of \(P\) on \(\partial \Omega _{4a}\) is zero (i.e. \(\nabla P \cdot {\varvec{t}}|_{\partial \Omega _{4a}}=0\)), since \(P=P^l\) on this section of boundary. It follows that \( \nabla P \cdot \mathbf n = |\nabla P|\) (here we expect \(\nabla P \cdot {\varvec{n}}>0\) since the pressure acts outward on the boundary). We can thus rewrite (69) as
Since both integrals can be integrated straightforwardly using CM the ODE for \(T_1(t)\) can be solved by CM. This allows \(u_c(t)\) to be determined, since the second integral term on the right-hand side of (67) is readily evaluated using CM.
Blood and Lymphatic Capillary, Boundaries \(\partial \Omega _{1}\) and \(\partial \Omega _{4}\)
Boundary conditions (30)–(31) can be implemented straightforwardly in CM using the Boundary Load feature, which describes a boundary that experiences zero tangential stresses but on which the normal stresses are balanced by an external pressure.
Rights and permissions
About this article
Cite this article
Heppell, C., Roose, T. & Richardson, G. A Model for Interstitial Drainage Through a Sliding Lymphatic Valve. Bull Math Biol 77, 1101–1131 (2015). https://doi.org/10.1007/s11538-015-0078-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-015-0078-4