Abstract
Polarization, whereby a cell defines a spatial axis by segregating specific determinants to distinct regions, is an essential and highly conserved biological process. The process of polarization is initiated by a cue that breaks an initially symmetric distribution of determinants, allowing for a spatially asymmetric redistribution. The nature of this cue is currently not well understood. Utilizing the conservation of polarization process and its determinants, we theoretically investigate the nature of the cue and the regulation of contractility that enables the establishment of polarity in early embryos of the nematode worm Caenorhabditis elegans (C. elegans). Our biologically based model, which consists of coupled partial differential equations, suggests that a biochemical but not mechanical cue is sufficient for symmetry breaking, and inhibition of contractile elements by specific determinants is needed for sustained spatial redistribution.















Similar content being viewed by others
References
Aranda V, Nolan ME, Muthuswamy SK (2008) Par complex in cancer: a regulator of normal cell polarity joins the dark side. Oncogene 27:6878–6887
Benton R, Johnston DS (2003) Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115:691–704
Carlsson AE (2006) Contractile stress generation by actomyosin gels. Phys Rev E Stat Nonlinear Soft Matter Phys 74:051912
Cheeks RJ, Canman JC, Gabriel WN, Meyer N, Strome S, Goldstein B, Hill C, Carolina N (2004) C. elegans PAR proteins function by mobilizing and stabilizing asymmetrically localized protein complexes. Curr Biol 14:851–862
Cowan CR, Hyman AA (2004) Asymmetric cell division in C. elegans: cortical polarity and spindle positioning. Annu Rev Cell Dev Biol 20:427–453
Cowan CR, Hyman AA (2004) Centrosomes direct cell polarity independently of microtubule assembly in C. elegans embryos. Nature 431:92–96
Cuenca AA, Schetter A, Aceto D, Kemphues K, Seydoux G (2003) Polarization of the C. elegans zygote proceeds via distinct establishment and maintenance phases. Development (Cambridge, England) 130:1255–1265
Czitrom V (1999) One-factor-at-a-time versus designed experiments. Am Stat 53:126–131
Dawes AT, Munro EM (2011) PAR-3 oligomerization may provide an actin-independent mechanism to maintain distinct Par protein domains in the early Caenorhabditis elegans embryo. Biophys J 101:1412–1422
Dawes AT, Iron D (2013) Cortical geometry may influence placement of interface between Par protein domains in early C. elegans embryos. J Theor Biol 333:27–37
Etienne-Manneville S, Hall A (2003) Cell polarity: Par6, aPKC and cytoskeletal crosstalk. Curr Opin Cell Biol 15:67–72
Feng W, Wu H, Chan L-N, Zhang M (2007) The Par-3 NTD adopts a PB1-like structure required for Par-3 oligomerization and membrane localization. EMBO J 26:2786–2796
Goehring NW, Trong PK, Bois JS, Chowdhury D, Nicola EM, Hyman AA, Grill SW (2011) Polarization of PAR proteins by advective triggering of a pattern-forming system. Science (New York, N.Y.) 334:1137–1141
Goehring NW, Hoege C, Grill SW, Hyman AA (2011) PAR proteins diffuse freely across the anterior-posterior boundary in polarized C. elegans embryos. J Cell Biol 193:583–594
Hao Y, Boyd L, Seydoux G (2006) Stabilization of cell polarity by the C. elegans RING protein PAR-2. Dev Cell 10:199–208
Hird SN, White JG (1993) Cortical and cytoplasmic flow polarity in early embryonic cells of Caenorhabditis elegans. J Cell Biol 121:1343–1355
Hoege C, Hyman AA (2013) Principles of PAR polarity in Caenorhabditis elegans embryos. Nature reviews. Mol Cell Biol 14:315–322
Hung TJ, Kemphues KJ (1999) PAR-6 is a conserved PDZ domain-containing protein that colocalizes with PAR-3 in Caenorhabditis elegans embryos. Development (Cambridge, England) 126:127–135
Hurd TW, Fan S, Liu CJ, Kweon HK, Hakansson K, Margolis B (2003) Phosphorylation-dependent binding of 14-3-3 to the polarity protein Par3 regulates cell polarity in mammalian epithelia. Curr Biol 13:2082–2090
Hurov JB, Watkins JL, Piwnica-Worms H (2004) Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr Biol 14:736–741
Iglesias PA, Devreotes PN (2012) Biased excitable networks: how cells direct motion in response to gradients. Curr Opin Cell Biol 24:245–253
Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, Tabuse Y, Kemphues KJ, Ohno S (1998) An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol 143:95–106
Jilkine A, Edelstein-Keshet L (2011) A comparison of mathematical models for polarization of single eukaryotic cells in response to guided cues. PLoS Comput Biol 7:e1001121
Joberty G, Petersen C, Gao L, Macara IG (2000) The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol 2:531–539
Kay AJ, Hunter CP (2001) CDC-42 regulates PAR protein localization and function to control cellular and embryonic polarity in C. elegans. Curr Biol 11:474–481
Klein TJ, Mlodzik M (2005) Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol 21:155–176
Li B, Kim H, Beers M, Kemphues K (2010) Different domains of C. elegans PAR-3 are required at different times in development. Dev Biol 344:745–757
Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T (2000) A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol 2:540–547
Marston DJ, Goldstein B (2006) Symmetry breaking in C. elegans: another gift from the sperm. Dev Cell 11:273–274
Motegi F, Sugimoto A (2006) Sequential functioning of the ECT-2 RhoGEF, RHO-1 and CDC-42 establishes cell polarity in Caenorhabditis elegans embryos. Nat Cell Biol 8:978–985
Motegi F, Zonies S, Hao Y, Cuenca AA, Griffin E, Seydoux G (2011) Microtubules induce self-organization of polarized PAR domains in Caenorhabditis elegans zygotes. Nat Cell Biol 13:1361–1367
Munro E, Nance J, Priess JR (2004) Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev Cell 7:413–424
Schonegg S, Hyman AA (2006) CDC-42 and RHO-1 coordinate acto-myosin contractility and PAR protein localization during polarity establishment in C. elegans embryos. Development (Cambridge, England) 133(18):3507–3516
Severson AF, Bowerman B (2003) Myosin and the PAR proteins polarize microfilament-dependent forces that shape and position mitotic spindles in Caenorhabditis elegans. J Cell Biol 161:21–26
Shelton CA, Carter JC, Ellis GC, Bowerman B (1999) The nonmuscle myosin regulatory light chain gene mlc-4 is required for cytokinesis, anterior–posterior polarity, and body morphology during Caenorhabditis elegans embryogenesis. J Cell Biol 146:439–451
Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T, Ohno S (2001) Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol 152:1183–1196
Suzuki A, Ohno S (2006) The PAR-aPKC system: lessons in polarity. J Cell Sci 119:979–987
Tervonen TA, Partanen JI, Saarikoski ST, Myllynen M, Marques E, Paasonen K, Moilanen A, Wohlfahrt G, Kovanen PE, Klefstrom J (2011) Faulty epithelial polarity genes and cancer. Adv Cancer Res 111:97–161
Thompson BJ (2013) Cell polarity: models and mechanisms from yeast, worms and flies. Development (Cambridge, England) 140:13–21
Tostevin F, Howard M (2008) Modeling the establishment of PAR protein polarity in the one-cell C. elegans embryo. Biophys J 95:4512–4522
Tsai M-C, Ahringer J (2007) Microtubules are involved in anterior–posterior axis formation in C. elegans embryos. J Cell Biol 179:397–402
Wallenfang MR, Seydoux G (2000) Polarization of the anterior-posterior axis of C. elegans is a microtubule-directed process. Nature 408:89–92
Yamanaka T, Horikoshi Y, Suzuki A, Sugiyama Y, Kitamura K, Maniwa R, Nagai Y, Yamashita A, Hirose T, Ishikawa H, Ohno S (2001) PAR-6 regulates aPKC activity in a novel way and mediates cell-cell contact-induced formation of the epithelial junctional complex. Genes Cells Devoted Mol Cell Mech 6:721–731
Zonies S, Motegi F, Hao Y, Seydoux G (2010) Symmetry breaking and polarization of the C. elegans zygote by the polarity protein PAR-2. Development (Cambridge, England) 137:1669–1677
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Model Nondimensionalization
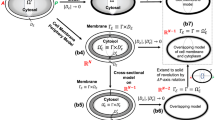
We base the model used here on previous Par proteins models (Dawes and Munro 2011; Dawes and Iron 2013). We assume that ParA can dimerize, and that ParA and ParP interact through mutual phosphorylation. We assume that ParA can enhance actomyosin assembly while ParP inhibits it. We assume that all proteins diffuse in the plane of the cortex and that actomyosin can be modeled as a viscoelastic substance where local tension gradients induce advective movement, making advection an emergent property of the model. Please see Dawes and Iron (2013) for further details on the kinetic and spatial terms. Incorporating diffusion and advection, the full dimensional model studied here is:
As discussed in Sect. 2.2, we do not know many of the kinetic rates associated with the interactions modeled by our equations. As a result, we non-dimensionalize the equations so that our variables can only take on values between 0 and 1, and our parameters can be expressed in terms of groupings of rate constants. We make the following substitution for our dependent and independent variables: \(C=\bar{C}C^*\) where \(C=A,P,M,t,x\), and \(\bar{C}\) is the characteristic (dimensional) scale for that variable, and \(C^*\) is the dimensionless variable. We define the following characteristic scales:
After substitution and simplification (and dropping the \(^*\)), we have Eqs. 1–5 with the parameter groupings given in Table 1.
Appendix 2: Simplification of Anterior Par Protein Model
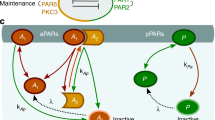
Anterior Par proteins have been shown to form higher-order complexes, and this oligomer formation is required for proper polarization in a variety of cell types (Joberty et al. 2000; Lin et al. 2000; Yamanaka et al. 2001; Izumi et al. 1998; Etienne-Manneville and Hall 2003; Suzuki et al. 2001; Li et al. 2010). In our previous models of Par protein dynamics, we explicitly modeled the binding and interconversion of the various possible states of the anterior Par proteins (Dawes and Munro 2011; Dawes and Iron 2013). By distilling down the interaction network as described below, we can reduce the dynamics of the anterior Par proteins to one variable.
The original anterior Par protein model consists of three equations, tracking the change over time of cortically bound ParA monomers, singly bound ParA dimers (where only one part of the dimer is bound to the cortex) and doubly bound dimers (both parts of the dimer are bound to the cortex). Please see Dawes and Munro (2011), Dawes and Iron (2013) for a more detailed discussion of the motivation, assumptions and equations behind this model. Here, we distill that model to its fundamental core: two ParA monomers in the cytoplasm bind to a cortical site, forming a dimer. We can express those interactions as the following arrow diagram:
where \(A_y\) is the number of cytoplasmic ParA monomers, \(B\) is the number of cortical binding sites, and \(A\) is the number of cortically bound ParA dimers. The number of cortical binding sites was implicitly included in the previous model, by having a maximum number of monomers and dimers that could bind to the cortex at any time. This is a biologically reasonable assumption, since steric interactions will prevent an unlimited number of ParA dimers to bind to the cortex, and suggests the first conservation law: The total number of cortical binding sites is constant. In other words,
Similarly, by having a maximum number of ParA monomers and dimers bound to the cortex, this suggests the total number of ParA monomers and dimers is conserved. That is,
Note here we are considering the number of monomers and dimers, and not concentrations. This simplifies the model by not having to include a volume conversion term for converting between the cortical and cytoplasmic concentrations.
Assuming mass-action kinetics and using conservation laws 20 and 21, the simplified one variable equation for \(A\) is:
However, we are also interested in spatial dynamics in addition to the kinetics. We observed that the actomyosin profile strongly mirrors the ParA profile during simulations. Thus, for this simplified model, we assume that actomyosin is proportional to ParA, allowing us to write the resulting one variable spatial model for \(A\) as:
After non-dimensionalizing as in Appendix 1, using \(\bar{A}=A_T,\,\tau =\frac{1}{k_{\mathrm{off}}^A},\, \bar{x}=L\), we have
where
Rights and permissions
About this article
Cite this article
Kravtsova, N., Dawes, A.T. Actomyosin Regulation and Symmetry Breaking in a Model of Polarization in the Early Caenorhabditis elegans Embryo. Bull Math Biol 76, 2426–2448 (2014). https://doi.org/10.1007/s11538-014-0016-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-014-0016-x