Abstract
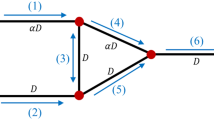
Numerical simulations of unsteady blood flow through a honeycomb network originating at multiple inlets and terminating at multiple outlets are presented and discussed under the assumption that blood behaves as a continuum with variable constitution. Unlike a tree network, the honeycomb network exhibits both diverging and converging bifurcations between branching capillary segments. Numerical results based on a finite difference method demonstrate that as in the case of tree networks considered in previous studies, the cell partitioning law at diverging bifurcations is an important parameter in both steady and unsteady flow. Specifically, a steady flow may spontaneously develop self-sustained oscillations at critical conditions by way of a Hopf bifurcation. Contrary to tree-like networks comprised entirely of diverging bifurcations, the critical parameters for instability in honeycomb networks depend weakly on the system size. The blockage of one or more network segments due to the presence of large cells or the occurrence of capillary constriction may cause flow reversal or trigger a transition to unsteady flow.










Similar content being viewed by others
References
Barber JO, Alberding JP, Restrepo JM, Secomb TW (2008) Simulated two-dimensional red blood cell motion, deformation, and partitioning in microvessel bifurcations. Ann Biomed Eng 36:1690–1698
Carr RT, Geddes JB, Wu F (2005) Oscillations in a simple microvascular network. Ann Biomed Eng 33:764–771
Carr RT, Lacoin M (2000) Nonlinear dynamics of microvascular blood flow. Ann Biomed Eng 28:641–652
Chiba A, Suzuki OY, Nakanishi H, Chichibu S (1998) Gastric vascular network in stroke-prone spontaneously hypertensive rats. Res Commun Mol Pathol Pharmacol 100:65–76
Davis JM, Pozrikidis C (2011) Numerical simulation of unsteady blood flow through capillary networks. Bull Math Biol 73:1857–1880
Dellimore JW, Dunlop J, Canham PB (1983) Ratio of cells and plasma in blood flowing past branches in small plastic channels. Am J Physiol 244:H635–H643
Dunlop SA, Moore SR, Beazley LD (1997) Changing patterns of vasculature in the developing amphibian retina. J Exp Biol 200:2479–2492
Duvernoy HM, Delon S, Vannson JL (1981) Cortical blood vessels of the human brain. Brain Res Bull 7:519–579
Fabry ME, Kaul DK, Carmen R, Baez S, Rieder R, Nagel RL (1981) Some aspects of the pathophysiology of homozygous Hb CC erythrocytes. J Clin Invest 67:1284–1291
Fenton BM, Carr RT, Cokelet GR (1985a) Nonuniform red cell distribution in 20 to 100 \(\mu \)m bifurcations. Microvasc Res 29:103–126
Fenton BM, Wilson DW, Cokelet GR (1985b) Analysis of the effects of measured white blood cell entrance times on hemodynamics in a computer model of a microvascular bed. Pflügers Arch 403:396–401
Folkman J, Klagsbrun M (1987) Angiogenic factors. Science 23:442–447
Forouzan O, Yang X, Sosa JM, Burns JM, Shevkoplyas SS (2012) Spontaneous oscillations of capillary blood flow in artificial microvascular networks. Microvasc Res 84:123–132
Fung YC (1973) Stochastic flow in capillary blood vessels. Microvasc Res 5:34–48
Geddes JB, Carr RT, Karst NJ, Wu F (2007) The onset of oscillations in microvascular blood flow. SIAM J Appl Dyn Syst 6:694–727
Geddes JB, Carr RT, Wu F, Lao Y, Maher M (2010) Blood flow in microvascular networks: a study in nonlinear biology. Chaos 20:045123
Hirsch S, Reichold J, Schneider M, Székely G, Weber B (2012) Topology and hemodynamics of the cortical cerebrovascular system. J Cereb Blood Flow Metab 32:952–967
Iooss G, Joseph DD (1990) Elementary stability and bifurcation theory, 2nd edn. Springer, New York
Kiani MF, Cokelet GR, Sarelius IH (1993) Effect of diameter variability along a microvessel segment on pressure drop. Microvasc Res 45:219–232
Kiani MF, Pries AR, Hsu LL, Sarelius IH, Cokelet GR (1994) Fluctuations in microvascular blood flow parameters caused by hemodynamics mechanisms. Am J Physiol 266:H1822–H1828
Kiesslich R, Neurath MF (2011) Endomicroscopy for in vivo diagnosis of colorectal cancer. In: Waye JD, Rex DK, Williams CB (eds) Colonoscopy: principles and practice. Wiley, New York
Klitzman B, Johnson PC (1982) Capillary network geometry and red cell distribution in the hamster cremaster muscle. Am J Physiol 242 (Heart Circ Physiol 11), H211–H219
Lauwers F, Sassot F, Lauwers-Cances V, Puwanarajah P, Duvernow H (2008) Morphometry of the human cerebral cortex microcirculation: general characteristics and space-related profiles. Neuroimage 39:936–948
Obrist D, Weber B, Buck A, Jenny P (2010) Red blood cell distribution in simplified capillary networks. Philos Trans R Soc A 368:2897–2918
Pop SR, Richardson G, Waters SL, Jensen OE (2007) Shock formation and non-linear dispersion in a microvascular capillary network. Math Med Biol 24:379–400
Pozrikidis C (2009) Numerical simulation of blood flow through microvascular capillary networks. Bull Math Biol 71:1520–1541
Pozrikidis C, Davis JM (2013) Blood flow through capillary networks. In: Becker SM, Kuznetsov AV (eds) Transport in biological Media. Elsevier, Amsterdam
Pozrikidis C, Hill AI (2012) Conduction through a damaged honeycomb lattice. Int J Heat Mass Transf 55:2052–2061
Pries AR, Neuhaus D, Gaehtgens P (1992) Blood viscosity in tube flow: dependence on diameter and hematocrit. Am J Physiol Heart Circ Physiol 96:H1770–H1778
Pries AR, Secomb TW, Gaehtgens P (1996) Biophysical aspects of blood flow in the microvasculature. Cardiovasc Res 32:654–667
Pries AR, Secomb TW, Gebner T, Sperandio MB, Gross JF, Gaehtgens P (1994) Resistance to blood flow in microvessels in vivo. Circ Res 75:904–915
Pries AR, Secomb TW, Gaehtgens P, Gross JF (1990) Blood flow in microvascular networks. Experiments and simulation. Circ Res 67:826–834
Ribatti D, Vacca A, Roncali L, Dammacco F (1996) The chick embryo chorioallantoic membrane as a model for in vivo research on angiogenesis. Int J Dev Biol 40:1189–1197
Ribatti D (2008) Chick embryo chorioallantoic membrane as a useful tool to study angiogenesis. Int Rev Cell Mol Biol 270:181–224
Roberts BW, Olbricht WW (2006) The distribution of freely suspended particles at microfluidic bifurcations. AIChE J 52:199–206
Safaeian N, Sellier M, Davis T (2011) A computational model of hemodynamics parameters in cortical capillary networks. J Theor Biol 271:145–156
Schmid-Schönbein GW, Skalak R, Usami S, Chien S (1980) Cell distribution in capillary networks. Microvasc Res 19:18–44
Tawfik Y, Owens RG (2013) A mathematical and numerical investigation of the hemodynamical origins of oscillations in microvascular networks. Bull Math Biol 75:676–707
West JR (2011) Respiratory physiology, 9th edn. Lippincott, Williams, & Wilkins, Baltimore, MD
Yen RT, Fung YC (1978) Effect of velocity distribution on red cell distribution in capillary blood vessels. Am J Physiol 235(2):H251–H257
Author information
Authors and Affiliations
Corresponding author
Appendix: Governing Equations
Appendix: Governing Equations
The discharge hematocrit, \(H_\mathrm{D}\), is the ratio of the volumetric flow rate of the red blood cells, \(Q_\mathrm{rbc}\), to that of the whole blood, \(Q\): \(H_\mathrm{D}\equiv Q_\mathrm{rbc}/{Q}\). The tube hematocrit, \(H_\mathrm{T}\), is the volume fraction of the suspended red blood cells inside the tube and differs from the discharge hematocrit, \(H_\mathrm{D}\), because the blood cell velocity is higher than the mean plasma velocity in capillary flow. The ratio of the tube hematocrit to the discharge hematocrit is related to the mean cell velocity, \(V_\mathrm{rbc}\), by \(\chi \equiv H_\mathrm{T}/H_\mathrm{D} = U_m/V_\mathrm{rbc}\), where \(U_m\) is the mean blood velocity. Pries et al. (1990) proposed a correlation for the hematocrit ratio,
where the capillary radius, \(a_\mathrm{c}\), is measured in \(\upmu \)m.
Consider the flow of a suspension of red blood cells through a straight, rigid and circular tube of radius \(a_\mathrm{c}\) and length \(L\). Cell-free zones near the vessel walls are ignored, and the cells are assumed to be distributed uniformly over the cross section. A cell population balance over a differential control volume confined between two parallel plates normal to the \(x\) axis leads to a one-dimensional convection equation for the evolution of the discharge hematocrit in each segment (e.g., Davis and Pozrikidis 2011),
The flow of a suspension of cells through a circular capillary is assumed to follow Poiseuille’s law with an effective fluid viscosity \(\mu _\text {eff}\) determined by the volume fraction of the suspended cells and by the radius of the capillary. A pressure difference \(\Delta P\) across the segment end nodes produces a flow rate
The effective blood viscosity, \(\mu _\text {eff}\), is regarded as a proportionality coefficient relating the flow rate to the pressure gradient and incorporates deviations from a circular cross section in each segment, imperfections, and entrance effects. Integrating Eq. (9) along the length of the capillary provides an expression for the mean velocity of the suspension,
where
is an averaged viscosity.
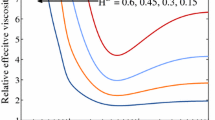
The effective viscosity of blood in a capillary segment depends on the capillary radius and hematocrit. Pries et al. (1994, 1996) proposed a correlation based on in vivo observations,
where \(\beta \equiv [a_\mathrm{c}/(a_\mathrm{c}-0.55) ]^2\). The capillary radius, \(a_\mathrm{c}\), is measured in \(\upmu \)m, \(\mu = 1.85\) cP is the plasma viscosity, \(C = [ \, 0.8+\exp (-0.15 \, a_\mathrm{c}) \,] \, (-1+f^{-1})+f^{-1}\) is a dimensionless exponent, \(f=1 + 10 \, (0.20 a_\mathrm{c})^{12}\), and
is the relative blood viscosity at \(H_\mathrm{D}=0.45\).
Consider a bifurcation node of a capillary network where one parental capillary segment divides into two descendent segments or two capillary segments converge into one segment. The local configuration involves four pressure nodes representing network junctions that form the end points of the three segments. The dynamics at a bifurcation is governed by mass and population balances. A mass balance requires that \(\sum _{i=1}^3 Q_i = 0\), which, using Eq. (9), can be expressed as
where \(P_0\) is the pressure at the central node, \({a_\mathrm{c}}_i\) is the radius of capillary \(i\), and \(Q_i\) is the corresponding flow rate. By convention, \(Q_i >0\) when blood is driven away from the bifurcation along the \(i\)th capillary for \(i=1, 2, 3\).
Conservation of the volume of the red blood cells at a bifurcation requires that
where the discharge hematocrit is evaluated at the end of the segment at the bifurcation. In the case of a converging bifurcation where, for example, \(Q_1<0,\,Q_2<0\),and \(Q_3>0\), the first two streams merge into one and Eq. (15) can be solved for \(H_{\mathrm{D}_3}\) in terms of the known \(H_{\mathrm{D}_1},\,H_{\mathrm{D}_2}\), and the three flow rates,
where
are positive fractional flow rates determined by hydrodynamics.
In the case of a diverging bifurcation where, for example, \(Q_1>0,\,Q_2>0\), and \(Q_3<0\), the third stream splits into two streams carrying fluid away from the bifurcation, and the red blood cell concentration in each descendent segment depends on the corresponding fractional flow rates. This dependence is incorporated into the model as an effective boundary condition for \(H_\mathrm{D}\) at the entrance of the descendent segments at the bifurcation. Introducing the positive cell fractional flow rates
the outgoing discharge hematocrits are given by
The cell partitioning law at a bifurcation can be written in terms of the partition function \(\phi _1/\phi _2 \equiv \mathcal {F}_1(a_3, H_{\mathrm{D}_3}; \psi _2, a_1/a_2)\) (e.g., Pozrikidis 2009). Consequently,
Klitzman and Johnson (1982) proposed a simple partitioning law,
where \(q\) is an unspecified, dimensionless parameter. When \(q=1\), all involved hematocrits are equal, \(H_{\mathrm{D}_1} = H_{\mathrm{D}_2} = H_{\mathrm{D}_3}\), independent of the individual flow rates. As \(q \rightarrow \infty \), all red blood cells are channeled into the descendent branch with the largest flow rate. Pries et al. (1990, 1992) proposed the empirical relation
where \(q= 1+ 3.49 \, (1-H_{\mathrm{D}_3})/a_3,\,X_0=0.2/a_3\), and the capillary radii, \(a_{1}, a_{2}\), and \(a_{3}\), are measured in \(\upmu \)m. When \(a_1=a_2\) and \(X_0=0\), the functional form of Eq. (21) is recovered. The partitioning law of Fenton et al. (1985b) corresponds to
where \(b_0 = 1/[1.4 - \sqrt{D_\mathrm{rbc}/(2a_3)}] \ge 1\) and \(D_\mathrm{rbc}\) is the diameter of a red blood cell.
Rights and permissions
About this article
Cite this article
Davis, J.M., Pozrikidis, C. Self-sustained Oscillations in Blood Flow Through a Honeycomb Capillary Network. Bull Math Biol 76, 2217–2237 (2014). https://doi.org/10.1007/s11538-014-0002-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-014-0002-3