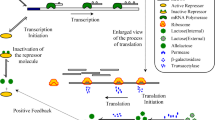
Abstract
In this work, we develop a detailed, stochastic, dynamical model for the tryptophan operon of E. coli, and estimate all of the model parameters from reported experimental data. We further employ the model to study the system performance, considering the amount of biochemical noise in the trp level, the system rise time after a nutritional shift, and the amount of repressor molecules necessary to maintain an adequate level of repression, as indicators of the system performance regime. We demonstrate that the level of cooperativity between repressor molecules bound to the first two operators in the trp promoter affects all of the above enlisted performance characteristics. Moreover, the cooperativity level found in the wild-type bacterial strain optimizes a cost-benefit function involving low biochemical noise in the tryptophan level, short rise time after a nutritional shift, and low number of regulatory molecules.





Similar content being viewed by others
References
Angulo-Brown, F., Santillán, M., & Calleja-Quevedo, E. (1995). Thermodynamic optimality in some biochemical reactions. Nuovo Cimento D, 17, 87–90.
Angulo-Brown, F., Maya, G., & Santillán, M. (2001). Local stability analysis of an endoreversible Curzon-Ahborn-Novikov engine working in a maximum-power-like regime. J. Phys. D, Appl. Phys., 34, 2068–2072. http://www.iop.org/EJ/abstract/0022-3727/34/13/318.
Arvidson, D. N., Bruce, C., & Gunsalus, R. P. (1986). Interaction of the Escherichia coli trp aporepressor with its ligand, L-tryptophan. J. Biol. Chem., 261, 238–243.
Baker, R., & Yanofsky, C. (1972). Transcription initiation frequency and translational yield for the tryptophan operon of Escherichia coli. J. Mol. Biol., 69, 89–102.
Bennett, B. D., Kimball, E. H., Gao, M., Osterhout, R., Van Dien, S. J., & Rabinowitz, J. D. (2009). Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat. Chem. Biol., 5, 593–599.
Bhartiya, S., Rawool, S., & Venkatesh, K. V. (2003). Dynamic model of Escherichia coli tryptophan operon shows an optimal structural design. Eur. J. Biochem., 270, 2644–2651.
Bliss, R. D., Painter, P. R., & Marr, A. G. (1982). Role of feedback inhibition in stabilizing the classical operon. J. Theor. Biol., 97, 177–193.
Bremer, H., & Dennis, P. P. (1996). Modulation of chemical composition and other parameters of the cell by growth rate. In Escherichia coli and Salmonella: cellular and molecular biology (Vol. 2, pp. 1553–1569).
Brown, M. P., Grillo, A. O., Boyer, M., & Royer, C. A. (1999). Probing the role of water in the tryptophan repressor-operator complex. Protein Sci., 8, 1276–1285.
Cai, X. (2007). Exact stochastic simulation of coupled chemical reactions with delays. J. Chem. Phys., 126, 124108.
Caligiuri, M. G., & Bauerle, R. (1991). Identification of amino acid residues involved in feedback regulation of the anthranilate synthase complex from Salmonella typhimurium. J. Biol. Chem., 266, 8328–8335.
Cartwright, K. V., Russell, P., & Kaminsky, E. J. (2012). Finding the maximum magnitude response (gain) of second-order filters without calculus. Lat. Am. J. Phys. Educ., 6, 559–565.
Chubukov, V., Zuleta, I. A., & Li, H. (2012). Regulatory architecture determines optimal regulation of gene expression in metabolic pathways. Proc. Natl. Acad. Sci., 109, 5127–5132.
De Boer, H. A., Comstock, L. J., & Vasser, M. The tac promoter: a functional hybrid derived from the trp and lac promoters. (1983). Proc. Natl. Acad. Sci., 80, 21.
Dekel, E., & Alon, U. (2005). Optimality and evolutionary tuning of the expression level of a protein. Nature, 436, 588–592.
Deuschle, U., Kammerer, W., Gentz, R., & Bujard, H. (1986). Promoters of Escherichia coli: a hierarchy of in vivo strength indicates alternate structures. EMBO J., 5, 2987.
Díaz-Hernández, O., Páez-Hernández, R., & Santillán, M. (2010). Thermodynamic performance vs. dynamic stability in an enzymatic reaction model. Physica A, 389, 3476–3483.
Dublanche, Y., Michalodimitrakis, K., Kuemmerer, N., Foglierini, M., & Serrano, L. (2006). Noise in transcription negative feedback loops: simulation and experimental analysis. Mol. Syst. Biol., 2, 41.
Forchhammer, J., Jackson, E. N., & Yanofsky, C. (1972). Different half-lives of messenger RNA corresponding to different segments of the tryptophan operon of Escherichia coli. J. Mol. Biol., 71, 687–699.
Gillespie, D. T. (1977). Exact stochastic simulation of coupled chemical reactions. J. Phys. Chem., 81, 2340–2361.
Grillo, A. O., Brown, M. P., & Royer, C. A. (1999). Probing the physical basis for trp repressor-operator recognition. J. Mol. Biol., 287, 539–554.
Gunsalus, R. P., Miguel, A. G., & Gunsalus, G. L. (1986). Intracellular Trp repressor levels in Escherichia coli. J. Bacteriol., 167, 272–278.
Hernández-Valdez, A., Santillán, M., & Zeron, E. S. (2010). Cycling expression and cooperative operator interaction in the trp operon of Escherichia coli. J. Theor. Biol., 263, 340–352.
Hurlburt, B. K., & Yanofsky, C. (1992). Analysis of heterodimer formation by the Escherichia coli trp repressor. J. Biol. Chem., 267, 16783–16789.
Ito, J., Cox, E. C., & Yanofsky, C. (1969). Anthranilate synthetase, an enzyme specified by the tryptophan operon of Escherichia coli: comparative studies on the complex and the subunits. J. Bacteriol., 97, 725–733.
Jardetzky, O., & Finucane, M. (2007). Tandem interactions in the trp repressor system may regulate binding to operator DNA. Struct. Biophys. New Technol. Curr. Chall. Biol. Beyond, 49–64.
Javelle, A., Thomas, G., Marini, A. M., Krämer, R., & Merrick, M. (2005). In vivo functional characterization of the Escherichia coli ammonium channel AmtB: evidence for metabolic coupling of AmtB to glutamine synthetase. Biochem. J., 390, 215.
Jeeves, M., Evans, P. D., Parslow, R. A., Jaseja, M., & Hyde, E. I. (1999). Studies of the Escherichia coli Trp repressor binding to its five operators and to variant operator sequences. Eur. J. Biochem., 265, 919–928.
Kennell, D., & Riezman, H. (1977). Transcription and translation initiation frequencies of the Escherichia coli lac operon. J. Mol. Biol., 114, 1–21.
Klipp, E., Heinrich, R., & Holzhütter, H.-G. (2002). Prediction of temporal gene expression. Eur. J. Biochem., 269, 5406–5413.
Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2008). Lehninger principles of biochemistry (5th ed.). New York: W.H. Freeman.
Mackey, M. C., & Santillán, M. (2008). Dynamic stability versus thermodynamic performance in a simple model for a Brownian motor. Phys. Rev. E, 78, 1–7.
Morse, D. E., Baker, R. F., & Yanofsky, C. (1968). Translation of the tryptophan messenger RNA of Escherichia coli. Proc. Natl. Acad. Sci. USA, 60, 1428–1435.
Neidhardt, F. C., Ingraham, J. L., & Schaechter, M. (1990). Physiology of the bacterial cell: a molecular approach. Sunderland: Sinauer Associates.
Oyarzún, D., Ingalls, B., Middleton, R., & Kalamatianos, D. (2009). Sequential activation of metabolic pathways: a dynamic optimization approach. Bull. Math. Biol., 71, 1851–1872.
Páez-Hernández, R., & Santillán, M. (2008). Comparison of the energetic properties and the dynamical stability in a mathematical model of the stretch reflex. Physica A, 387, 3574–3582.
Páez-Hernández, R., Angulo-Brown, F., & Santillán, M. (2006). Dynamic robustness and thermodynamic optimization in a non-endoreversible Curzon–Ahlborn engine. J. Non-Equilib. Thermodyn., 31, 173–188.
Poelwijk, F. J., de Vos, M. G., & Tans, S. J. (2011). Tradeoffs and optimality in the evolution of gene regulation. Cell, 146, 462–470.
Quan, S., Ray, J., Kwota, Z., Duong, T., Balázsi, G., Cooper, T. F., & Monds, R. D. (2012). Adaptive evolution of the lactose utilization network in experimentally evolved populations of Escherichia coli. PLoS Genet., 8, e1002444.
Raj, A., & van Oudenaarden, A. (2009). Single-molecule approaches to stochastic gene expression. Annu. Rev. Biophys., 38, 255–270.
Salazar-Cavazos, E., & Santillán, M. (2012). Transcriptional bursting in the tryptophan operon of E. coli and its effect on the system stochastic dynamics. In R. S. Sharma (Ed.), Enzyme inhibition and bioapplications (pp. 179–194). Rijeka: Intech.
Santillán, M., & Angulo-Brown, F. (1997). A thermodynamic approach to the compromise between power and efficiency in muscle contraction. J. Theor. Biol., 189, 391–398.
Santillán, M., & Zeron, E. S. (2004). Dynamic influence of feedback enzyme inhibition and transcription attenuation on the tryptophan operon response to nutritional shifts. J. Theor. Biol., 231, 287–298.
Santillán, M., Arias-Hernández, L. A., & Angulo-Brown, F. (1997). Some optimization criteria for biological systems in linear irreversible thermodynamics. Nuovo Cimento D, 19, 99–109.
Schmitt, T. H., Zheng, Z., & Jardetzky, O. (1995). Dynamics of tryptophan binding to Escherichia coli Trp repressor wild type and AV77 mutant: an NMR study. Biochemistry, 34, 13183–13189.
Shahrezaei, V., & Swain, P. S. (2008). Analytical distributions for stochastic gene expression. Proc. Natl. Acad. Sci., 105, 17256–17261.
Sinha, S. (1988). Theoretical study of tryptophan operon: applications in microbial technology. Biotechnol. Bioeng., 31, 117–124.
Tabaka, M., Cybulski, O., & Holyst, R. (2008). Accurate genetic switch in Escherichia coli: novel mechanism of regulation by co-repressor. J. Mol. Biol., 377, 1002–1014.
Taniguchi, Y., Choi, P. J., Li, G.-W., Chen, H., Babu, M., Hearn, J., Emili, A., & Xie, X. S. (2010). Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science, 329, 533–538.
van Hoek, M., & Hogeweg, P. (2007). The effect of stochasticity on the lac operon: an evolutionary perspective. PLoS Comput. Biol., 3, e111.
Wall, M. E., Hlavacek, W. S., & Savageau, M. A. (2003). Design principles for regulator gene expression in a repressible gene circuit. J. Mol. Biol., 332, 861–876.
Xie, G., Keyhani, N. O., Bonner, C. A., & Jensen, R. A. (2003). Ancient origin of the tryptophan operon and the dynamics of evolutionary change. Microbiol. Mol. Biol. Rev., 67, 303–342.
Yang, J., Gunasekera, A., Lavoie, T. A., Jin, L., Lewis, D. E. A., & Carey, J. (1996). In vivo and in vitro studies of TrpR-DNA interactions. J. Mol. Biol., 258, 37–52.
Yanofsky, C. (2000). Transcription attenuation, once viewed as a novel regulatory strategy. J. Bacteriol., 182, 1–8.
Yanofsky, C., & Crawford, I. P. (1987). The tryptophan operon. In F. C. Neidhart, J. L. Ingraham, K. B. Low, B. Magasanik, & H. E. Umbarger (Eds.), Escherichia coli and Salmonella thyphymurium: cellular and molecular biology (Vol. 2, pp. 1453–1472). Washington: Am. Soc. Microbiol.
Yanofsky, C., & Horn, V. (1994). Role of regulatory features of the trp operon of Escherichia coli in mediating a response to a nutritional shift. J. Bacteriol., 176, 6245–6254.
Yanofsky, C., Platt, T., Crawford, I. P., Nichols, B. P., Christie, G. E., Horowitz, H., VanCleemput, M., & Wu, A. M. (1981). The complete nucleotide sequence of the tryptophan operon of Escherichia coli. Nucleic Acids Res., 9, 6647–6668.
Yanofsky, C., Kelley, R. L., & Horn, V. (1984). Repression is relieved before attenuation in the trp operon of Escherichia coli as tryptophan starvation becomes increasingly severe. J. Bacteriol., 158, 1018–1024.
Zeron, E. S., & Santillán (2010). Distributions for negative-feedback-regulated stochastic gene expression: dimension reduction and numerical solution of the chemical master equation. J. Theor. Biol., 264, 377–385.
Zhang, H., Zhao, D., Revington, M., Lee, W., Jia, X., Arrowsmith, C., & Jardetzky, O. (1994). The solution structures of the trp repressor-operator DNA complex. J. Mol. Biol., 238, 592–614.
Acknowledgements
ES-C acknowledges financial support from Consejo Nacional de Ciencia y Tecnología (CONACyT) through scholarship No. 302844. MS thanks McGill University and particularly Prof. Michael C. Mackey for their hospitality during his sabbatical leave, during which part of the research for this paper was performed. Both authors are grateful with the anonymous reviewers whose comments greatly helped to improve this paper.
Author information
Authors and Affiliations
Corresponding author
Appendix: Parameter Estimation
Appendix: Parameter Estimation
In this work, we consider a cell volume of 1 μm3 (Javelle et al. 2005). On the other hand, from experimental measures made by Bennett et al. (2009) on E. coli growing in minimal medium with glucose, the bacterial average doubling time is 77 min. Then
Baker and Yanofsky (1972) calculated that during exponential growth E. coli possess around 1.8 copies of the trp operon. Therefore, the probabilities that a cell has 1 or 2 copies are 0.2 and 0.8, respectively. We developed an algorithm that use these probabilities to randomly choose the initial number of copies of the trp operon, and thus of the promoter. The initial state of the promoter(s) is selected by another algorithm that calculates and uses the probabilities of each of the 8 possible states at the initial levels of tryptophan.
From the work of Morse et al. (1968), the number of anthranilate synthase enzymes before de-repression is
On the other hand, we have from the website E. coli Statistics ( http://ccdb.wishartlab.com/CCDB/cgi-bin/STAT_NEW.cgi ) that the number of tryptophan molecules is
The dissociation constant of the reaction through which a tryptophan binds one of its binding sites at the repressor, as obtained by Arvidson et al. (1986), is
From the experimental results of Gunsalus et al. (1986), in which the number of repressors in E. coli cultured in medium without tryptophan was calculated, we have that
For the association propensities of the active repressor with the different operators (\(k_{i}^{+}\)) and the dissociation propensities of the repressor in its three different states (\(k_{i,R}^{-}\), \(k_{i,R_{T}}^{-}\), and \(k_{i,R_{2T}}^{-}\)), we used the values employed by Tabaka et al. (2008). These values, calculated from the experimental works of Grillo et al. (1999), Hurlburt and Yanofsky (1992), Zhang et al. (1994), and Jardetzky and Finucane (2007), are shown below:
The dissociation constant of the reaction through which a tryptophan molecule binds one of its binding sites in a repressor bound to an operator, K B , was recalculated from the work of Tabaka et al. (2008) using the K T value obtained by Arvidson et al. (1986), instead of the one estimated by Schmitt et al. (1995):
The constant accounting for the cooperative interaction between repressors bound to operators O 1 and O 2 was experimentally estimated by Yang et al. (1996):
We computed the propensity for transcription initiation at a non-repressed promoter from the reported transcription initiation rate of the lac operon, and from the comparative strengths of the trp, lacUV5 and lac promoters (Kennell and Riezman 1977; De Boer et al. 1983; Deuschle et al. 1986). The trp promoter resulted to be around 1.43 times stronger than the lac promoter, which has a maximal transcription rate of 18.2 molecules/min. Thus,
Yanofsky et al. (1984) found that transcriptional attenuation is relieved only when the intracellular concentration of tryptophan is extremely low, and that the probability that transcriptional attenuation occurs at these conditions is 6 times smaller in comparison to instances in which tryptophan concentration is higher. The values of parameters K G and α that allow Eq. (6) to represent this behavior are
To calculate the time between transcriptional initiation and the moment in which translation can start without being momentarily stopped by the RNA polymerase, τ M , we need to remember that the enzyme anthranilate synthase is produced from trpE and trpD genes. Knowing that the distance between the site of transcriptional initiation and the start codon of trpD gene is 1724 nucleotides (nt) and that the RNA polymerase in bacteria with a doubling time of 60 min advance at a speed of a speed of 2700 nt/min, we obtain that it will take 0.64 min for the polymerase to start transcribing gene trpD (Yanofsky et al. 1981; Bremer and Dennis 1996). On the other hand, if we consider that the ribosome advances at a slightly higher rate (2880 nt/min) than the RNA polymerase, and that the length of trpD gene is 1596 nt, we obtain that
Forchhammer et al. (1972) determined that the regions of the mRNA corresponding to the trpE and trpD genes have half-lives of 1 and 1.25 min, respectively. Using the shortest half-life, we have that the mRNA-degradation propensity is
To obtain the translation initiation propensity, k E , we used two experimentally determined values. The first one is the number of ribosomes that translate each mRNA from the trp operon in all its life time, which is approximately 30 (Baker and Yanofsky 1972). The second one is the mRNA mean lifetime, which can be calculated from γ M as 1.45 min. Thus,
Considering that the subunit of the enzyme anthranilate synthase that takes more time to be produced is TrpD, that the length of the trpD gene is 1,596 nt, and the ribosome elongation rate is 2,880 nt/min, we have
The propensity of tryptophan production by active enzyme was obtained from the experimental studies of Ito et al. (1969):
The values of the half saturation constant K I and the corresponding Hill coefficient n, where calculated by Caligiuri and Bauerle (1991):
To compute the maximum propensity of tryptophan consumption, γ, we took the number of tryptophan molecules incorporated in all the proteins of the cell and we divided it by the time in which the cell, and thus the proteins, are doubled. From the website E. coli Statistics we obtain that each E. coli bacteria has around 2.6 million proteins, each one with an average length of 360 amino acids. With this information, and knowing that the relative abundance of tryptophan with respect to all the amino acids is 1.1 %, there are around 10.3 million tryptophan molecules present in proteins (Neidhardt et al. 1990). If the doubling time is 77 min, then
To take into account that protein production, and consequently tryptophan consumption, decreases only when the number of tryptophan molecules in the cell is scarce we used
The system described in this work reaches stationary levels for the enzyme and tryptophan of around 1000 and 4100 molecules per bacterial cell, respectively. These values are in agreement with the experimental data of Bliss et al. (1982) and Bennett et al. (2009).
Rights and permissions
About this article
Cite this article
Salazar-Cavazos, E., Santillán, M. Optimal Performance of the Tryptophan Operon of E. coli: A stochastic, Dynamical, Mathematical-Modeling Approach. Bull Math Biol 76, 314–334 (2014). https://doi.org/10.1007/s11538-013-9920-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-013-9920-8