Abstract
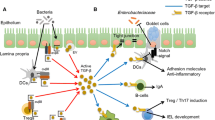
Gut mucosal homeostasis depends on complex interactions among the microbiota, the intestinal epithelium, and the gut associated immune system. A breakdown in some of these interactions may precipitate inflammation. Inflammatory bowel diseases, Crohn’s disease, and ulcerative colitis are chronic inflammatory disorders of the gastrointestinal tract. The initial stages of disease are marked by an abnormally high level of pro-inflammatory helper T cells, Th1. In later stages, Th2 helper cells may dominate while the Th1 response may dampen. The interaction among the T cells includes the regulatory T cells (Treg). The present paper develops a mathematical model by a system of differential equations with terms nonlocal in the space spanned by the concentrations of cytokines that represents the interaction among T cells through a cytokine signaling network. The model demonstrates how the abnormal levels of T cells observed in inflammatory bowel diseases can arise from abnormal regulation of Th1 and Th2 cells by Treg cells.





Similar content being viewed by others
References
Abraham, C., & Cho, J. H. (2009). Inflammatory bowel disease. N. Engl. J. Med., 361, 2066–2078.
Babyatsky, M. W., Rossiter, G., & Podolsky, D. K. (1996). Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology, 110, 975–984.
Bamias, G., Kaltsa, G., & Ladas, S. D. (2011). Cytokines in the pathogenesis of ulcerative colitis. Discov. Medicin., 11, 459–467.
Baumgart, D., & Carding, S. (2007). Inflammatory bowel disease: cause and immunobiology. Lancet, 369(9573), 1627–1640.
Bouma, G., & Strober, W. (2003). The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol., 3(7), 521–533.
Conlon, P. J., Tyler, S., Grabstein, K. H., & Morrissey, P. (1989–1990) Interleukin-4 (b-cell stimulatory factor-1) augments the in vivo generation of cytotoxic cells in immunosuppressed animals. Biotechnol. Ther., 1, 31–41.
Cosmi, L., Liotta, F., Angeli, R., Mazzinghi, B., Santarlasci, V., Manetti, R., Lasagni, L., Vanini, V., Romagnani, P., Maggi, E., Annunziato, F., & Romagnani, S. (2004). Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines. Blood, 103, 3117–3121.
Czarniecki, C. W., & Sonnenfeld, G. (1993). Interferon-gamma and resistance to bacterial infections. APMIS, Acta Pathol. Microbiol. Immunol. Scand., 101, 1–17.
Eastaff-Leung, N., Mabarrack, N., Barbour, A., Cummins, A., & Barry, S. (2010). Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J. Clin. Immunol., 30, 80–89.
Germain, R. N. (2012). Maintaining system homeostasis: the third law of Newtonian immunology. Nat. Immunol., 13, 902–906.
Grassegger, A., & Hopf, R. (2004). Significance of the cytokine interferon gamma in clinical dermatology. Clin. Exp. Dermatol., 29, 584–588.
Grogan, J. L., Mohrs, M., Harmon, B., Lacy, D. A., Sedat, J. W., & Locksley, R. M. (2001). Early transcription and silencing of cytokine genes underlie polarization of t helper cell subsets. Immunity, 14(3), 205–215.
Gross, F., Metzner, G., & Behn, U. (2010). Mathematical modeling of allergy and specific immunotherapy: Th1–Th2–Treg interactions. J. Theor. Biol., 269, 70–78.
Hofer, T., Nathansen, H., Lohning, M., Radbruch, A., & Heinrich, R. (2002). GATA-3 transcriptional imprinting in Th2 lymphocytes: a mathematical model. Proc. Natl. Acad. Sci. USA, 99, 9364–9368.
Hwang, E. S., Hong, J. H., & Glimcher, L. H. (2005). IL-2 production in developing Th1 cells is regulated by heterodimerization of RelA and T-bet and requires T-bet serine residue 508. J. Exp. Med., 202, 1289–1300.
Ishikawa, D., Okazawa, A., Corridoni, D., Jia, L. G., Wang, X. M., Guanzon, M., Xin, W., Arseneau, K. O., Pizarro, T. T., & Cominelli, F. (2012). Tregs are dysfunctional in vivo in a spontaneous murine model of Crohn’s disease. Mucosal Immunol. doi:10.1038/mi.2012.67.
Kaminskam, B., Wesolowska, A., & Danilkiewicz, M. (2005). Tgf beta signalling and its role in tumour pathogenesis. Acta Biochim. Pol., 52, 329–337.
Kato, K., Fukunaga, K., Kamikozuru, K., Kashiwamura, S., Hida, N., Ohda, Y., Takeda, N., Yoshida, K., Iimuro, M., Yokoyama, Y., Kikuyama, R., Miwa, H., & Matsumoto, T. (2011). Infliximab therapy impacts the peripheral immune system of immunomodulator and corticosteroid naive patients with Crohn’s disease. Gut Liver, 5, 37–45.
Kugathasan, S., Saubermann, L. J., Smith, L., Kou, D., Itoh, J., Binion, D. G., Levine, A. D., Blumberg, R. S., & Fiocchi, C. (2007). Mucosal T-cell immunoregulation varies in early and late inflammatory bowel disease. Gut, 56, 1696–1705.
Lee, S.-M., Gao, B., & Fang, D. (2008). Foxp3 maintains Treg unresponsiveness by selectively inhibiting the promoter DNA-binding activity of AP-1. Blood, 111(7), 3599–3606.
Lighvani, A. A., Frucht, D. M., Jankovic, D., Yamane, H., Aliberti, J., Hissong, B. D., Nguyen, B. V., Gadina, M., Sher, A., Paul, W. E., & O’Shea, J. J. (2001). T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc. Natl. Acad. Sci. USA, 98(15), 137–142.
Maloy, K., & Powrie, F. (2011). Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature, 474(7351), 298–306.
Matsuoka, K., Inoue, N., Sato, T., Okamoto, S., Hisamatsu, T., Kishi, Y., Sakuraba, A., Hitotsumatsu, O., Ogata, H., Koganei, K., Fukushima, T., Kanai, T., Watanabe, M., Ishii, H., & Hibi, T. (2004). T-bet upregulation and subsequent interleukin 12 stimulation are essential for induction of Th1 mediated immunopathology in Crohn’s disease. Gut, 53, 1303–1308.
Maul, J., Loddenkemper, C., Mundt, P., Berg, E., Giese, T., Stallmach, A., Zeitz, M., & Duchmann, R. (2005). Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology, 128, 1868–1878.
Mitsuyama, K., Tomiyasu, N., Takaki, K., Masuda, J., Yamasaki, H., Kuwaki, K., Takeda, T., Kitazaki, S., Tsuruta, O., & Sata, M. (2006). Interleukin-10 in the pathophysiology of inflammatory bowel disease: increased serum concentrations during the recovery phase. Mediat. Inflamm., 2006(6), 26875.
Nelson, B. H. (2004). IL-2, regulatory T cells, and tolerance. J. Immunol., 172, 3983–3988.
Osugi, Y., Hara, J., Tagawa, S., Takai, K., Hosoi, G., Matsuda, Y., Ohta, H., Fujisaki, H., Kobayashi, M., Sakata, N., Kawa-Ha, K., Okada, S., & Tawa, A. (1997). Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood, 89, 4100–4103.
Pak, S., Holland, N., Garnett, E. A., Mileti, E., Mahadevan, U., Beckert, R., Kanwar, B., & Heyman, M. B. (2012). Cytokine profiles in peripheral blood of children and adults with Crohn disease. J. Pediatr. Gastroenterol. Nutr., 54, 769–775.
Rao, B. M., Driver, I., Lauffenburger, D. A., & Wittrup, K. D. (2004). Interleukin 2 (IL-2) variants engineered for increased IL-2 receptor alpha-subunit affinity exhibit increased potency arising from a cell surface ligand reservoir effect. Mol. Pharmacol., 66, 864–869.
Robinson, T. M., Nelson, R. G., & Boyer, J. D. (2003). Parasitic infection and the polarized Th2 immune response can alter a vaccine-induced immune response. DNA Cell Biol., 22, 421–430.
Rubin, D. T., Uluscu, O., & Sederman, R. (2012). Response to biologic therapy in Crohn’s disease is improved with early treatment: An analysis of health claims data. Inflamm. Bowel Dis. doi:10.1002/ibd.22925.
Sakaguchi, S., Wing, K., & Yamaguchi, T. (2009). Dynamics of peripheral tolerance and immune regulation mediated by Treg. Eur. J. Immunol., 39, 2331–2336.
Sansonetti, P. J. (2004). War and peace at mucosal surfaces. Nat. Rev. Immunol., 4, 953–964.
Stummvoll, G. H., DiPaolo, R. J., Huter, E. N., Davidson, T. S., Glass, D., Ward, J. M., & Shevach, E. M. (2008). Th1, Th2, and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. J. Immunol., 181(3), 1908–1916.
Szabo, S. J., Dighe, A. S., Gubler, U., & Murphy, K. M. (1997). Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med., 185(5), 817–824.
Wendelsdorf, K., Bassaganya-Riera, J., Hontecillas, R., & Eubank, S. (2010). Model of colonic inflammation: immune modulatory mechanisms in inflammatory bowel disease. J. Theor. Biol., 264, 1225–1239.
Yates, A., Callard, R., & Stark, J. (2004). Combining cytokine signalling with T-bet and GATA-3 regulation in Th1 and Th2 differentiation: a model for cellular decision-making. J. Theor. Biol., 231(2), 181–196.
Zenewicz, L. A., Antov, A., & Flavell, R. A. (2009). CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol. Med., 15(5), 199–207.
Zhu, J., Jankovic, D., Oler, A., Wei, G., Sharma, S., Hu, G., Guo, L., Yagi, R., Yamane, H., Punkosdy, G., Feigenbaum, L., Zhao, K., & Paul, W. E. (2012). The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity, 37, 660–673.
Acknowledgements
This research has been supported by the Mathematical Biosciences Institute and the National Science Foundation under Grant DMS 0931642.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lo, WC., Arsenescu, R.I. & Friedman, A. Mathematical Model of the Roles of T Cells in Inflammatory Bowel Disease. Bull Math Biol 75, 1417–1433 (2013). https://doi.org/10.1007/s11538-013-9853-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-013-9853-2