Abstract
The parasite Trypanosoma cruzi, known for causing Chagas’ disease, is spread via insect vectors from the triatomine family. T. cruzi is maintained in sylvatic vector-host transmission cycles in certain parts of the Americas. Communication between the cycles occurs mainly through movement (migration) of the insect vectors. In this study, we develop a cellular automaton (CA) model in order to study invasion of a hypothetical strain of T. cruzi through the region defined by the primary sylvatic cycles in northern Mexico and parts of the southeastern United States. The model given is a deterministic CA, which can be described as a large metapopulation model in the format of a dynamical system with 9,376 equations. The migration rates in the model, used as coupling parameters between cells in the CA, are estimated by summing up the proportion of vectors crossing patch boundaries (i.e., crossing from one cell to another). Specifically, we develop methods for estimating speed and direction of invasion as a function of vector migration rates, including preference for a particular direction of migration. We develop two methods for estimating invasion speed: via orthogonal local velocity components and by direct computation of magnitude and direction of an overall velocity vector given a front created by cells identified as being invaded by the epidemic. Results indicate that invasion speed is greatly affected by both the physical and the epidemiological landscapes through which the infection wave passes. A power-law fit suggests that invasion speed increases at slightly less than the square root of increases in migration rate.


















Similar content being viewed by others
References
Crawford, B. A., & Kribs-Zaleta, C. M. (2013a). Vector migration and dispersal rates for sylvatic T. cruzi transmission. Ecol. Complex.. doi:10.1016/j.ecocom.2012.11.003.
Crawford, B. A., & Kribs-Zaleta, C. M. (2013b, in press). A metapopulation model for T. cruzi sylvatic transmission with vector migration. Math. Biosci. Eng.
Devillers, H., Lobry, J. R., & Menu, F. (2008). An agent-based model for predicting the prevalence of Trypanosoma cruzi I and II in their host and vector populations. J. Theor. Biol., 255, 307–315.
Dorn, P., Buekens, P., Hanford, E. (2007). Whac-a-mole: future trends in Chagas disease transmission and a global perspective on disease control. Futur. Microbiol., 2, 365–367.
Fisher, R. A. (1937). The wave of advance of advantageous genes. Ann. Eugen., 7, 355–369.
Fu, S., & Milne, G. (2003). Epidemic modelling using cellular automata. In Proceedings of the Australian conference on artificial life.
Huang, W., Han, M., & Liu, K. (2010). Dynamics of an SIS reaction-diffusion epidemic model for disease transmission. Math. Biosci. Eng., 7, 51–66.
Kot, M., Lewis, M. A., & van den Driessche, P. (1996). Dispersal data and the spread of invading organisms. Ecology, 77, 2027–2042.
Kribs Zaleta, C. (2010). Estimating contact process saturation in sylvatic transmission of Trypanosoma cruzi in the U.S. PLOS Neglected Trop. Dis., 4(4), e656. pp. 1–14.
Lambert, R. C., Kolivras, K. N., Resler, L. M., Brewster, C. C., & Paulson, S. L. (2008). The potential for emergence of Chagas disease in the United States. Geospatial Health, 2, 227–239.
Lewis, M., Renclawowicz, J., & van den Driessche, P. (2006). Traveling waves and spread rates for a West Nile virus model. Bull. Math. Biol., 68, 3–23.
Medlock, J., & Kot, M. (2003). Spreading disease: integro-differential equations old and new. Math. Biosci., 184, 201–222.
Mollison, D. (1977). Spatial contact models for ecological and epidemic spread. J. R. Stat. Soc. B, 39, 283–326.
Murray, J. D., Stanley, E. A., & Brown, D. L. (1986). On the spatial spread of rabies among foxes. Proc. R. Soc. Lond., 229, 111–1150.
Roellig, D. M., Brown, E. L., Barnabé, C., Tibayrenc, M., Steurer, F. J., & Yabsley, M. J. (2008). Molecular typing of Trypanosoma cruzi isolates, United States. Emerg. Infect. Dis., 14, 1123–1125.
Schönfisch, B. (1995). Propagation of fronts in cellular automata. Physica D, 80, 433–450.
Skellam, J. G. (1951). Random dispersal in theoretical populations. Biometrika, 38, 196–218.
Slimi, R., El Yacoubi, S., Dumonteil, E., & Gourbière, S. (2009). A cellular automata model for Chagas disease. Appl. Math. Model., 33, 1072–1085.
Sirakoulis, G. C., Karafyllidis, I., & Thanailakis, A. (2000). A cellular automaton model for the effects of population movement and vaccination on epidemic propagation. Ecol. Model., 133, 209–223.
Sun, G. Q., Liu, Q. X., Jin, Z., Chakraborty, A., & Li, B. L. (2010). Influence of infection rate and migration on extinction of disease in spatial epidemics. J. Theor. Biol., 624, 95–103.
White, S., Martín del Rey, A., & Rodríguez Sánchez, G. (2007). Modeling epidemics using cellular automata. Appl. Comput. Math., 186, 193–202.
Yabsley, M., Barnes, J., Ellise, A., Kjos, S., & Roxanne, C. (2012). Southern plains woodrats (Neotoma micropus) from southern Texas are important reservoirs of two genotypes of Trypanosoma cruzi and a host of a putative novel Trypanosoma species. Vector-Borne Zoonotic Dis., 13, 1–9.
Acknowledgements
This research was supported by a 2008 Norman Hackerman Advanced Research Program grant, and by the National Science Foundation under Grant DMS-1020880. Conclusions are those of the authors, and do not reflect the opinions of the funding sources. The authors also wish to acknowledge Christopher Hall and Michael Yabsley for discussions of the underlying biology, and Gaik Ambartsoumian and James Grover for suggestions which improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendix: Equations and Assumptions
Appendix: Equations and Assumptions
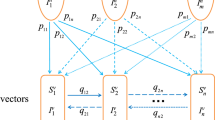
The system contains 9,376 equations. Each cell contains anywhere from 4 to 8 equations, depending on the specific patch location. The equations in (1) are representative of a patch 2 interior cell, which contains both species of vector and both hosts. Each state variable contains two subscripts: one identifying the species (S-T. sanguisuga, G-T. gerstaeckeri, R-raccoon, W-woodrat) and the other representing the cell location (i represents the current cell, while n, s, e, w represents the 4 possible adjacent cells (north, south, east, and west) to the current cell. Each vector equation contains 8 migration terms, representing the bidirectional movement between cells assuming a von Neumann radius. Not every equation in every cell will contain all migration terms. Specific migration terms will be 0 if there is no corresponding vector species population in the specific adjacent cell or if the current cell is on a grid boundary. The parameter definitions and values are given in Table 11.
Rights and permissions
About this article
Cite this article
Crawford, B.A., Kribs-Zaleta, C.M. & Ambartsoumian, G. Invasion Speed in Cellular Automaton Models for T. cruzi Vector Migration. Bull Math Biol 75, 1051–1081 (2013). https://doi.org/10.1007/s11538-013-9840-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-013-9840-7