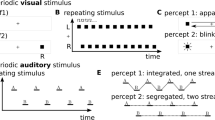Abstract
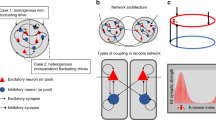
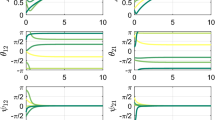
We analyze a competitive neural network model of perceptual rivalry that receives time-varying inputs. Time-dependence of inputs can be discrete or smooth. Spike frequency adaptation provides negative feedback that generates network oscillations when inputs are constant in time. Oscillations that resemble perceptual rivalry involve only one population being “ON” at a time, which represents the dominance of a single percept at a time. As shown in Laing and Chow (J. Comput. Neurosci. 12(1):39–53, 2002), for sufficiently high contrast, one can derive relationships between dominance times and contrast that agree with Levelt’s propositions (Levelt in On binocular rivalry, 1965). Time-dependent stimuli give rise to novel network oscillations where both, one, or neither populations are “ON” at any given time. When a single population receives an interrupted stimulus, the fundamental mode of behavior we find is phase-locking, where the temporally driven population locks its state to the stimulus. Other behaviors are analyzed as bifurcations from this forced oscillation, using fast/slow analysis that exploits the slow timescale of adaptation. When both populations receive time-varying input, we find mixtures of fusion and sole population dominance, and we partition parameter space into particular oscillation types. Finally, when a single population’s input contrast is smoothly varied in time, 1:n mode-locked states arise through period-adding bifurcations beyond phase-locking. Our results provide several testable predictions for future psychophysical experiments on perceptual rivalry.















Similar content being viewed by others
References
Abbott, L. F., Varela, J. A., Sen, K., & Nelson, S. B. (1997). Synaptic depression and cortical gain control. Science, 275(5297), 220–224.
Amari, S.-I. (1977). Dynamics of pattern formation in lateral-inhibition type neural fields. Biol. Cybern., 27(2), 77–87.
Benda, J., & Herz, A. V. M. (2003). A universal model for spike-frequency adaptation. Neural Comput., 15(11), 2523–2564.
Blake, R. (1989). A neural theory of binocular rivalry. Psychol. Rev., 96(1), 145–167.
Blake, R., & Logothetis, N. (2002). Visual competition. Nat. Rev., Neurosci., 3, 1–11.
Blake, R., Sobel, K. V., & Gilroy, L. A. (2003). Visual motion retards alternations between conflicting perceptual interpretations. Neuron, 39(5), 869–878.
Bossink, C. J., Stalmeier, P. F., & De Weert, C. M. (1993). A test of Levelt’s second proposition for binocular rivalry. Vis. Res., 33(10), 1413–1419.
Brascamp, J. W., van Ee, R., Noest, A. J., Jacobs, R. H. A. H., & van den Berg, A. V. (2006). The time course of binocular rivalry reveals a fundamental role of noise. J. Vis., 6(11), 1244–1256.
Brascamp, J. W., Knapen, T. H. J., Kanai, R., Noest, A. J., van Ee, R., & van den Berg, A. V. (2008). Multi-timescale perceptual history resolves visual ambiguity. PLoS ONE, 3(1), e1497.
Brascamp, J. W., Pearson, J., Blake, R., & van den Berg, A. V. (2009). Intermittent ambiguous stimuli: implicit memory causes periodic perceptual alternations. J. Vis., 9(3), 3.1.
Buckthought, A., Kim, J., & Wilson, H. R. (2008). Hysteresis effects in stereopsis and binocular rivalry. Vis. Res., 48(6), 819–830.
Chen, X., & He, S. (2004). Local factors determine the stabilization of monocular ambiguous and binocular rivalry stimuli. Curr. Biol., 14(11), 1013–1017.
Coombes, S., & Bressloff, P. C. (1999). Mode locking and Arnold tongues in integrate-and-fire neural oscillators. Phys. Rev. E, 60(2 Pt B), 2086–2096.
Curtu, R. (2010). Singular Hopf bifurcations and mixed-mode oscillations in a two-cell inhibitory neural network. Physica D, 239(9), 504–514.
Curtu, R., Shpiro, A., Rubin, N., & Rinzel, J. (2008). Mechanisms for frequency control in neuronal competition models. SIAM J. Appl. Dyn. Syst., 7(2), 609–649.
Fender, D., & Julesz, B. (1967). Extension of Panum’s fusional area in binocularly stabilized vision. J. Opt. Soc. Am., 57(6), 819–830.
Gigante, G., Mattia, M., Braun, J., & Del Giudice, P. (2009). Bistable perception modeled as competing stochastic integrations at two levels. PLoS Comput. Biol., 5(7), e1000430.
Harrad, R. A., McKee, S. P., Blake, R., & Yang, Y. (1994). Binocular rivalry disrupts stereopsis. Perception, 23(1), 15–28.
Kang, M. S., Heeger, D., & Blake, R. (2009). Periodic perturbations producing phase-locked fluctuations in visual perception. J. Vis., 9(2), 8.1.
Kilpatrick, Z. P., & Bressloff, P. C. (2010). Binocular rivalry in a competitive neural network with synaptic depression. SIAM J. Appl. Dyn. Syst., 9(4), 1303–1347.
Klink, P. C., van Ee, R., Nijs, M. M., Brouwer, G. J., Noest, A. J., & van Wezel, R. J. A. (2008). Early interactions between neuronal adaptation and voluntary control determine perceptual choices in bistable vision. J. Vis., 8(5), 16.1.
Laing, C. R., & Chow, C. C. (2002). A spiking neuron model for binocular rivalry. J. Comput. Neurosci., 12(1), 39–53.
Lankheet, M. J. M. (2006). Unraveling adaptation and mutual inhibition in perceptual rivalry. J. Vis., 6(4), 304–310.
Leopold, D. A., Wilke, M., Maier, A., & Logothetis, N. K. (2002). Stable perception of visually ambiguous patterns. Nat. Neurosci., 5(6), 605–609.
Levelt, W. J. M. (1965). On binocular rivalry. Institute for Perception RVO–TNO, Soesterberg, The Netherlands.
Moreno-Bote, R., Rinzel, J., & Rubin, N. (2007). Noise-induced alternations in an attractor network model of perceptual bistability. J. Neurophysiol., 98(3), 1125–1139.
Moreno-Bote, R., Shpiro, A., Rinzel, J., & Rubin, N. (2010). Alternation rate in perceptual bistability is maximal at and symmetric around equi-dominance. J. Vis., 10(11), 1.1.
Mueller, T., & Blake, R. (1989). A fresh look at the temporal dynamics of binocular rivalry. Biol. Cybern., 61, 223–232.
Noest, A. J., van Ee, R., Nijs, M. M., & van Wezel, R. J. A. (2007). Percept-choice sequences driven by interrupted ambiguous stimuli: a low-level neural model. J. Vis., 7(8), 10.1.
Orbach, J., Ehrlich, D., & Heath, H. A. (1963). Reversibility of the Necker cube. I. An examination of the concept of “satiation of orientation.” Percept. Mot. Skills, 17, 439–458.
Pearson, J., & Brascamp, J. (2008). Sensory memory for ambiguous vision. Trends Cogn. Sci., 12(9), 334–341.
Seely, J., & Chow, C. C. (2011). Role of mutual inhibition in binocular rivalry. J. Neurophysiol., 106(5), 2136–2150.
Shpiro, A., Curtu, R., Rinzel, J., & Rubin, N. (2007). Dynamical characteristics common to neuronal competition models. J. Neurophysiol., 97(1), 462–473.
Suzuki, S., & Grabowecky, M. (2002). Evidence for perceptual “trapping” and adaptation in multistable binocular rivalry. Neuron, 36(1), 143–157.
Taylor, A. L., Cottrell, G. W., & Kristan, W. B. Jr. (2002). Analysis of oscillations in a reciprocally inhibitory network with synaptic depression. Neural Comput., 14(3), 561–581.
van Ee, R. (2011). Percept-switch nucleation in binocular rivalry reveals local adaptation characteristics of early visual processing. J. Vis., 11(2), 13.1.
Wang, X. J., & Rinzel, J. (1992). Alternating and synchronous rhythms in reciprocally inhibitory model neurons. Neural Comput., 4(1), 84–97.
Webster, M. A. (2011). Adaptation and visual coding. J. Vis., 11(5), 2.1.
Wilson, H. R. (2003). Computational evidence for a rivalry hierarchy in vision. Proc. Natl. Acad. Sci. USA, 100(24), 14499–14503.
Wilson, H. R., Blake, R., & Lee, S. H. (2001). Dynamics of travelling waves in visual perception. Nature, 412(6850), 907–910.
Wolfe, J. M. (1986). Stereopsis and binocular rivalry. Psychol. Rev., 93(3), 269–282.
Acknowledgements
S.J. was supported by an NSF Research Experience for Undergraduates Fellowship (EMSW21-RTG 0739261). Z.P.K. is supported by an NSF Mathematical Sciences Postdoctoral Research Fellowship (DMS-1004422).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayasuriya, S., Kilpatrick, Z.P. Effects of Time-Dependent Stimuli in a Competitive Neural Network Model of Perceptual Rivalry. Bull Math Biol 74, 1396–1426 (2012). https://doi.org/10.1007/s11538-012-9718-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-012-9718-0