Abstract
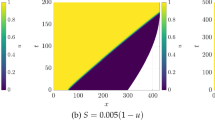
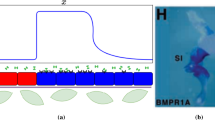
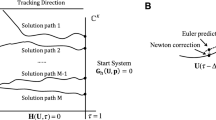
We investigate a reaction–diffusion system consisting of an activator and an inhibitor in a two-dimensional domain. There is a morphogen gradient in the domain. The production of the activator depends on the concentration of the morphogen. Mathematically, this leads to reaction–diffusion equations with explicitly space-dependent terms. It is well known that in the absence of an external morphogen, the system can produce either spots or stripes via the Turing bifurcation. We derive first-order expansions for the possible patterns in the presence of an external morphogen and show how both stripes and spots are affected. This work generalizes previous one-dimensional results to two dimensions. Specifically, we consider the quasi-one-dimensional case of a thin rectangular domain and the case of a square domain. We apply the results to a model of skeletal pattern formation in vertebrate limbs. In the framework of reaction–diffusion models, our results suggest a simple explanation for some recent experimental findings in the mouse limb which are much harder to explain in positional-information-type models.
Similar content being viewed by others
References
Ahn, S., & Joyner, A. L. (2004). Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell, 118, 505–516.
Alber, M. S., Glimm, T., Hentschel, H. G. E., Kazmierczak, B., & Newman, S. A. (2005). Stability of n-dimensional patterns in a generalized Turing system: implications for biological pattern formation. Nonlinearity, 18, 125–138.
Alber, M., Glimm, T., Hentschel, H. G. E., Kazmierczak, B., Zhang, Y.-T., Zhu, J., & Newman, S. A. (2008). The morphostatic limit for a model of skeletal pattern formation in the vertebrate limb. Bull. Math. Biol., 70(2), 460–483.
Bhat, R., Lerea, K. M., Peng, H., Kaltner, H., Gabius, H.-J., & Newman, S. A. (2011). A regulatory network of two galectins mediates the earliest steps of avian limb skeletal morphogenesis. BMC Dev. Biol., 11, 6.
Borckmans, A., De Wit, A., & Dewel, G. (1992). Competition in ramped Turing structures. Physica A, 188, 137–157.
Bouldin, C. M., Gritli-Linde, A., Ahn, S., & Harfe, B. D. (2010). Shh pathway activation is present and required within the vertebrate limb bud apical ectodermal ridge for normal autopod patterning. Proc. Natl. Acad. Sci. USA, 107, 5489–5494.
Cickovski, T. M., Huang, C., Chaturvedi, R., Glimm, T., Hentschel, H. G., Alber, M. S., Glazier, J. A., Newman, S. A., & Izaguirre, J. A. (2005). A framework for three-dimensional simulation of morphogenesis. IEEE/ACM Trans. Comput. Biol. Bioinform., 2, 273–288.
Ermentrout, B. (1991). Stripes or spots? Nonlinear effects in bifurcation of reaction–diffusion equations on the square. Proc. R. Soc. Lond. Ser. A, 434(1891), 413–417.
Forgacs, G., & Newman, S. (2005). Biological physics of the developing embryo. Cambridge, UK: Cambridge University Press.
Glimm, T., Zhang, J., & Shen, Y.-Q. (2009). Interaction of Turing patterns with an external linear morphogen gradient. Nonlinearity, 22(10), 2541–2560.
Harfe, B. D., Scherz, P. J., Nissim, S., Tian, H., McMahon, A. P., & Tabin, C. J. (2004). Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell, 118, 517–528.
Hentschel, H. G. E., Glimm, T., Glazier, J.A., & Newman, S.A. (2004). Dynamical mechanisms for skeletal pattern formation in the vertebrate limb. Proc. R. Soc. Lond. B, Biol. Sci., 271(1549), 1713–1722.
Klopocki, E., Ott, C. E., Benatar, N., Ullmann, R., Mundlos, S., & Lehmann, K. (2008). A microduplication of the long range SHH limb regulator (ZRS) is associated with triphalangeal thumb-polysyndactyly syndrome. J. Med. Genet., 45, 370–375.
Miura, T., Shiota, K., Morriss-Kay, G., & Maini, P. K. (2006). Mixed-mode pattern in doublefoot mutant mouse limb-Turing reaction–diffusion model on a growing domain during limb development. J. Theor. Biol., 240, 562–573.
Murray, J. D. (2002). Interdisciplinary applied mathematics: Vol. 17. Mathematical biology. I (3rd ed.). New York: Springer.
Newman, S. A., & Bhat, R. (2007). Activator-inhibitor dynamics of vertebrate limb pattern formation. Birth Defects Res. C, Embryo Today, 81, 305–319.
Newman, S. A., & Frisch, H. L. (1979). Dynamics of skeletal pattern formation in developing chick limb. Science, 205, 662–668.
Newman, S. A., Christley, S., Glimm, T., Hentschel, H. G., Kazmierczak, B., Zhang, Y. T., Zhu, J., & Alber, M. (2008). Multiscale models for vertebrate limb development. Curr. Top. Dev. Biol., 81, 311–340.
Page, K., Maini, P. K., & Monk, N. A. M. (2003). Pattern formation in spatially heterogeneous Turing reaction–diffusion models. Physica D, 181(1-2), 80–101.
Page, K. M., Maini, P. K., & Monk, N. A. M. (2005). Complex pattern formation in reaction–diffusion systems with spatially varying parameters. Physica D, 202(1–2), 95–115.
Riddle, R. D., Johnson, R. L., Laufer, E., & Tabin, C. (1993). Sonic hedgehog mediates the polarizing activity of the ZPA. Cell, 75, 1401–1416.
Sick, S., Reinker, S., Timmer, J., & Schlake, T. (2006). WNT and DKK determine hair follicle spacing through a reaction–diffusion mechanism. Science, 314, 1447–1450.
Sun, M., Ma, F., Zeng, X., Liu, Q., Zhao, X. L., Wu, F. X., Wu, G. P., Zhang, Z. F., Gu, B., Zhao, Y. F., Tian, S. H., Lin, B., Kong, X. Y., Zhang, X. L., Yang, W., Lo, W. H., & Zhang, X. (2008). Triphalangeal thumb-polysyndactyly syndrome and syndactyly type IV are caused by genomic duplications involving the long range, limb-specific SHH enhancer. J. Med. Genet., 45, 589–595.
Tickle, C. (2006). Making digit patterns in the vertebrate limb. Nat. Rev. Mol. Cell Biol., 7, 45–53.
Towers, M., & Tickle, C. (2009). Generation of pattern and form in the developing limb. Int. J. Dev. Biol., 53, 805–812.
Towers, M., Mahood, R., Yin, Y., & Tickle, C. (2008). Integration of growth and specification in chick wing digit-patterning. Nature, 452, 882–886.
Turing, A. M. (1952). The chemical basis of morphogenesis. Philos. Trans. R. Soc. B, 237, 37–72.
Wieczorek, D., Pawlik, B., Li, Y., Akarsu, N. A., Caliebe, A., May, K. J., Schweiger, B., Vargas, F. R., Balci, S., Gillessen-Kaesbach, G., & Wollnik, B. (2010). A specific mutation in the distant sonic hedgehog (SHH) cis-regulator (ZRS) causes Werner mesomelic syndrome (WMS) while complete ZRS duplications underlie Haas type polysyndactyly and preaxial polydactyly (PPD) with or without triphalangeal thumb. Hum. Mutat. 31, 81–89.
Wolpert, L., Beddington, R., Brockes, J., & Meyerowitz, E. (2002). Principles of development. London: Oxford University Press.
Zeller, R., Lopez-Rios, J., & Zuniga, A. (2009). Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat. Rev. Genet., 10, 845–858.
Zeng, X., Goetz, J. A., Suber, L. M., Scott, W. J., Schreiner, C. M., & Robbins, D. J. (2001). A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature, 411, 716–720.
Zhu, J., Zhang, Y. T., Alber, M. S., & Newman, S. A. (2010). Bare bones pattern formation: a core regulatory network in varying geometries reproduces major features of vertebrate limb development and evolution. PLoS ONE, 5, e10892.
Author information
Authors and Affiliations
Corresponding author
Additional information
Glimm and Zhang are joint first authors.
Rights and permissions
About this article
Cite this article
Glimm, T., Zhang, J., Shen, YQ. et al. Reaction–Diffusion Systems and External Morphogen Gradients: The Two-Dimensional Case, with an Application to Skeletal Pattern Formation. Bull Math Biol 74, 666–687 (2012). https://doi.org/10.1007/s11538-011-9689-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-011-9689-6