Abstract
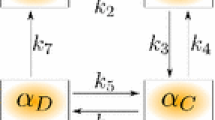
We extend and analyze the Wang and Politi modified Hai–Murphy model of smooth muscle cell contractions to capture uterine muscle cell response to variations in intracellular calcium concentrations. This model is used to estimate values of unknown parameters in uterine smooth muscle cell cross-bridging. Uterine motility is responsible for carrying out important processes throughout all phases of the nonpregnant female reproductive cycle, including sperm transport, menstruation, and embryo implantation. The modified Hai–Murphy partial differential equation model accounts for the displacement of myosin cross-bridge heads relative to their binding sites. This model was originally developed for the study of airway contractions; we now extended it for use in modeling nonisometric uterine contractions. Our extended model incorporates cross-bridge position and contractile velocity into the original model, resulting in more accurate modeling of the initial stages of contraction and modeling nonisometric contractions. Numerical simulations show that the contraction rate in our extended model is faster than the original Hai–Murphy model. These simulations provide quantitative estimates for the increased level of responsiveness of our extended model to intracellular calcium concentrations. The extended model and new parameter estimates for the cross-bridging can be coupled with uterine flow models to advance our understanding of embryonic motility and intrauterine flow.
Similar content being viewed by others
References
Ali, F., Pare, P. D., & Seow, C. Y. (2005). Models of contractile units and their assembly in smooth muscle. Can. J. Physiol. Pharm., 83(10), 825–831. LR: 20061115; JID: 0372712; 0 (Actins); EC 3.6.1.4 (Myosins); RF: 40; ppublish.
Burdyga, T., Borisova, L., Burdyga, A. T., & Wray, S. (2009). Temporal and spatial variations in spontaneous Ca events and mechanical activity in pregnant rat myometrium. Eur. J. Obstet., Gynecol., Reprod. Biol., 144(Suppl. 1), S25–S32. Supplement: Reproductive Bioengineering 2008.
Bursztyn, L., Eytan, O., Jaffa, A. J., & Elad, D. (2007). Mathematical model of excitation-contraction in a uterine smooth muscle cell. Am. J. Physiol., Cell Physiol., 292(5), C1816–C1829.
Hai, C. M., & Murphy, R. A. (1988). Cross-bridge phosphorylation and regulation of latch state in smooth muscle. Am. J. Physiol., 254(1 Pt 1), C99–C106. LR: 20071114; GR: HL-19242/HL/NHLBI NIH HHS/United States; JID: 0370511; EC 2.7.1.117 (Myosin-Light-Chain Kinase); EC 3.6.1.4 (Myosins); ppublish.
Herrera, A. M., McParland, B. E., Bienkowska, A., Tait, R., Paré, P. D., & Seow, C. Y. (2005). Sarcomeres of smooth muscle: functional characteristics and ultrastructural evidence. J. Cell Sci., 118, 2381–2392.
Huxley, A. F. (1957). Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem., 7, 255–318.
JT, F., RM, S., & JA, S. (1994). Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature, 368, 113–119.
Keener, J., & Sneyd, J. (2008). Mathematical physiology. Berlin: Springer (2nd edn.).
Kunz, G., Deininger, H., Wildt, L., & Leyendecker, G. (1996). The dynamics of rapid sperm transport through the female genital tract: evidence from vaginal sonography of uterine peristalsis and hysterosalpingoscintigraphy. Hum. Reprod., 11, 627–632.
Kupittayanant, S., Luckas, M., & Wray, S. (2002). Effect of inhibiting the sarcoplasmic reticulum on spontaneous and oxytocin-induced contractions of human myometrium. BJOG: Int. J. Obstet. Gynaecol., 109(3), 289–296.
Leyendecker, G., Kunz, G., Wildt, L., Beil, D., & Deininger, H. (1996). Uterine hyperperistalsis and dysperistalsis as dysfunctions of the mechanism of rapid sperm transport in patients with endometriosis and infertility. Hum. Reprod., 11(7), 1542–1551.
Lyons, E., Taylor, P., & Zheng, X. H. (1991). Characterization of subendometrial myometrial contractions throughout the menstrual cycle in normal fertile women. Fertil. Steril., 55(4), 771–774.
Mardon, H., Grewal, S., & Mills, K. (2007). Experimental models for investigating implantation of the human embryo. Semin. Reprod. Med., 25, 410–417.
Murphy, R. (1994). What is special about smooth muscle? The significance of covalent crossbridge regulation. FASEB J., 8(3), 311–318.
Parkington, H. C., Tonta, M. A., Brennecke, S. P., & Coleman, H. A. (1999). Contractile activity membrane potential, and cytoplasmic calcium in human uterine smooth muscle in the third trimester of pregnancy and during labor. Am. J. Obstet. Gynecol., 181(6), 1445–1451.
Shmigol, A. V., Eisner, D. A., & Wray, S. (2001). Simultaneous measurements of changes in sarcoplasmic reticulum and cytosolic [Ca2+] in rat uterine smooth muscle cells. J. Physiol., 531(3), 707–713.
Shmygol, A., Blanks, A. M., Bru-Mercier, G., Gullam, J. E., & Thornton, S. (2007). Control of uterine Ca2+ by membrane voltage. Ann. N.Y. Acad. Sci., 1101, 97–109.
Tang, D. C., Stull, J. T., Kubota, Y., & Kamm, K. E. (1992). Regulation of the Ca2+ dependence of smooth muscle contraction. J. Biol. Chem., 267(17), 11839–11845.
Tonino, P., Simon, M., & Craig, R. (2002). Mass determination of native smooth muscle myosin filaments by scanning transmission electron microscopy. J. Mol. Biol., 318, 999–1007.
Wang, I., Politi, A. Z., Tania, N., Bai, Y., Sanderson, M. J., & Sneyd, J. (2008). A mathematical model of airway and pulmonary arteriole smooth muscle. Biophys. J., 94(6), 2053–2064. LR: 20090317; JID: 0370626; 7440-70-2 (Calcium); EC 3.1.3.53 (Myosin-Light-Chain Phosphatase); OID: NLM: PMC2257911; 2007/12/07 [aheadofprint]; ppublish.
Word, R., Tang, D., & Kamm, K. (1994). Activation properties of myosin light chain kinase during contraction/relaxation cycles of tonic and phasic smooth muscles. J. Biol. Chem., 269, 21596–21602.
Wray, S. (2007). Insights into the uterus. Exp. Physiol., 92(4), 621–631.
Yaniv, S., Jaffa, A. J., Eytan, O., & Elad, D. (2009). Simulation of embryo transport in a closed uterine cavity model. Eur. J. Obstet., Gynecol., Reprod. Biol., 144(Suppl. 1), S50–S60. Supplement: Reproductive Bioengineering 2008.
Young, R. C. (2007). Myocytes, myometrium, and uterine contractions. Ann. N.Y. Acad. Sci., 1101, 72–84. JID: 7506858; RF: 12; 2007/04/18 [aheadofprint]; ppublish.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maggio, C.D., Jennings, S.R., Robichaux, J.L. et al. A Modified Hai–Murphy Model of Uterine Smooth Muscle Contraction. Bull Math Biol 74, 143–158 (2012). https://doi.org/10.1007/s11538-011-9681-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-011-9681-1