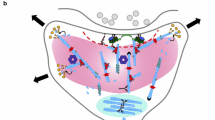
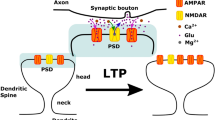
Abstract
Synaptic spines grow as a consequence of the formation of F-actin filaments at the spine head. The dynamics of F-actin in the spine head upon excitation of N-methy-D-aspartate (NMDA) receptors has recently been investigated experimentally, but there is no quantitative account of how these dynamic changes occur upon activation of these receptors; this we now supply. Dynamics of F-actin at the apex of lamellipodia have been investigated in detail, giving rise to the treadmilling theory of F-actin dynamics, involving catalysis by profilin, for which quantitative models are now available. Here, we adapt such a model to describe the dynamics of F-actin in the synaptic-spine head and show that it gives quantitative descriptions of this treadmilling phenomena which are well fitted by Monte Carlo simulations. Next, the means by which excitation of NMDA receptors enhances the activity of profilin through activity of the Rho small GTPase RhoA and the specific kinase ROCK is discussed. This is then used to model the NMDA receptor excitatory enhancement of profilin and so the treadmilling process of F-actin dynamics in spine growth. Such modelling provides a quantitative description of the synaptic-spine dynamics of the filamentous to globular actin ratio that is observed experimentally.
Similar content being viewed by others
References
Ahmed, R., Zha, X., Green, S., & Dailey, M. (2006). Synaptic activity and F-actin coordinately regulate CaMKIIα localization to dendritic postsynaptic sites in developing hippocampal slices. Mol. Cell. Neurosci., 31(1), 37–51.
Applewhite, D., Barzik, M., Kojima, S., Svitkina, T., Gertler, F., & Borisy, G. (2007). Ena/VASP proteins have an anti-capping independent function in filopodia formation. Mol. Biol. Cell, 18(7), 2579.
Asanuma, K., Kim, K., Oh, J., Giardino, L., Chabanis, S., Faul, C., Reiser, J., & Mundel, P. (2005). Synaptopodin regulates the actin-bundling activity of α-actin in an isoform-specific manner. J. Clin. Invest., 115(5), 1188–1198.
Asrican, B., Lisman, J., & Otmakhov, N. (2007). Synaptic strength of individual spines correlates with bound Ca2+ calmodulin-dependent kinase II. J. Neurosci., 27(51), 14007.
Bennett, M. (2009). Positive and negative symptoms in schizophrenia: the NMDA receptor hypofunction hypothesis neuregulin/ErbB4 and synapse regression. Aust. N. Z. J. Psychiatry, 43(8), 711–721.
Bramham, C., & Wells, D. (2007). Dendritic mRNA: transport, translation and function. Nat. Rev. Neurosci., 8(10), 776–789.
Buchs, P., & Muller, D. (1996). Induction of long-term potentiation is associated with major ultrastructural changes of activated synapses. Proc. Natl. Acad. Sci. USA, 93(15), 8040–8045.
Cai, Y., Biais, N., Giannone, G., Tanase, M., Jiang, G., Hofman, J., Wiggins, C., Silberzan, P., Buguin, A., Ladoux, B., et al. (2006). Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys. J., 91(10), 3907–3920.
Carlisle, H., & Kennedy, M. (2005). Spine architecture and synaptic plasticity. Trends Neurosci., 28(4), 182–187.
Carvalho, A., Caldeira, M., Santos, S., & Duarte, C. (2008). Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br. J. Pharmacol., 153(1), 310.
Cingolani, L., & Goda, Y. (2008). Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci., 9(5), 344–356.
Da Silva, J., Medina, M., Zuliani, C., Di Nardo, A., Witke, W., & Dotti, C. (2003). RhoA/ROCK regulation of neuritogenesis via profilin IIa-mediated control of actin stability. J. Cell Biol., 162(7), 1267.
Delorme, V., Machacek, M., DerMardirossian, C., Anderson, K., Wittmann, T., Hanein, D., Waterman-Storer, C., Danuser, G., & Bokoch, G. (2007). Cofilin activity downstream of Pak1 regulates cell protrusion efficiency by organizing lamellipodium and lamella actin networks. Dev. Cell, 13(5), 646–662.
Edwards, D., Sanders, L., Bokoch, G., & Gill, G. (1999). Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol., 1, 253–259.
Ethell, I., & Pasquale, E. (2005). Molecular mechanisms of dendritic spine development and remodeling. Prog. Neurobiol., 75(3), 161–205.
Fifkova, E., & Morales, M. (1989). Calcium-regulated contractile and cytoskeletal proteins in dendritic spines may control synaptic plasticity. Ann. NY Acad. Sci., 568(1), 131–137.
Haeckel, A., Ahuja, R., Gundelfinger, E., Qualmann, B., & Kessels, M. (2008). The actin-binding protein Abp1 controls dendritic spine morphology and is important for spine head and synapse formation. J. Neurosci., 28(40), 10031.
Higgs, H., & Pollard, T. (2001). Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Ann. Rev. Biochem., 70(1), 649–676.
Honkura, N., Matsuzaki, M., Noguchi, J., Ellis-Davies, G., & Kasai, H. (2008). The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron, 57(5), 719–729.
Hosokawa, T., Rusakov, D., Bliss, T., & Fine, A. (1995). Repeated confocal imaging of individual dendritic spines in the living hippocampal slice: evidence for changes in length and orientation associated with chemically induced LTP. J. Neurosci., 15(8), 5560–5573.
Huber, F., Käs, J., & Stuhrmann, B. (2008). Growing actin networks form lamellipodium and lamellum by self-assembly. Biophys. J., 95(12), 5508–5523.
Lin, C., Espreafico, E., Mooseker, M., & Forscher, P. (1997). Myosin drives retrograde F-actin flow in neuronal growth cones. Biol. Bull. 192, 1, 183–185.
Mogilner, A. (2006). On the edge: modeling protrusion. Curr. Opin. Cell Biol., 18(1), 32–39.
Mogilner, A., & Edelstein-Keshet, L. (2002). Regulation of actin dynamics in rapidly moving cells: a quantitative analysis. Biophys. J., 83(3), 1237–1258.
Nakagawa, T., Engler, J., & Sheng, M. (2004). The dynamic turnover and functional roles of α-actinin in dendritic spines. Neuropharmacology, 47(5), 734–745.
Nakayama, A., & Luo, L. (2000). Intracellular signaling pathways that regulate dendritic spine morphogenesis. Hippocampus, 10(5).
Okamoto, K., Nagai, T., Miyawaki, A., & Hayashi, Y. (2004). Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat. Neurosci., 7(10), 1104–1112.
Okamoto, K., Narayanan, R., Lee, S., Murata, K., & Hayashi, Y. (2007). The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc. Natl. Acad. Sci. USA, 104(15), 6418.
Okubo-Suzuki, R., Okada, D., Sekiguchi, M., & Inokuchi, K. (2008). Synaptopodin maintains the neural activity-dependent enlargement of dendritic spines in hippocampal neurons. Mol. Cell. Neurosci., 38(2), 266–276.
Pollard, T., & Borisy, G. (2003). Cellular motility driven by assembly and disassembly of actin filaments. Cell, 112(4), 453–465.
Racz, B., & Weinberg, R. (2006). Spatial organization of cofilin in dendritic spines. Neuroscience, 138(2), 447–456.
Racz, B., & Weinberg, R. (2008). Organization of the Arp2/3 complex in hippocampal spines. J. Neurosci., 28(22), 5654.
Rohm, B., Rahim, B., Kleiber, B., Hovatta, I., & Püschel, A. (2000). The semaphorin 3A receptor may directly regulate the activity of small GTPases. FEBS Lett., 486(1), 68–72.
Rostaing, P., Real, E., Siksou, L., Lechaire, J., Boudier, T., Boeckers, T., Gertler, F., Gundelfinger, E., Triller, A., & Marty, S. (2006). Analysis of synaptic ultrastructure without fixative using high-pressure freezing and tomography. Eur. J. Neurosci., 24(12), 3463.
Saneyoshi, T., Wayman, G., Fortin, D., Davare, M., Hoshi, N., Nozaki, N., Natsume, T., & Soderling, T. (2008). Activity-dependent synaptogenesis: regulation by a CaM-kinase kinase/CaM-kinase I/βPIX signaling complex. Neuron, 57(1), 94–107.
Schaus, T., Taylor, E., & Borisy, G. (2007). Self-organization of actin filament orientation in the dendritic-nucleation/array-treadmilling model. Proc. Natl. Acad. Sci. USA, 104(17), 7086.
Schmidt, J., Morgan, P., Dowell, N., & Leu, B. (2002). Myosin light chain phosphorylation and growth cone motility. J. Neurobiol., 52(3).
Schubert, V., & Dotti, C. (2007). Transmitting on actin: synaptic control of dendritic architecture. J. Cell Sci., 120(2), 205.
Schubert, V., Da Silva, J., & Dotti, C. (2006). Localized recruitment and activation of RhoA underlies dendritic spine morphology in a glutamate receptor-dependent manner. J. Cell Biol., 172(3), 453.
Sharma, K., Fong, D., & Craig, A. (2006). Postsynaptic protein mobility in dendritic spines: long-term regulation by synaptic NMDA receptor activation. Mol. Cell. Neurosci., 31(4), 702–712.
Shen, K., Teruel, M., Subramanian, K., & Meyer, T. (1998). CaMKII functions as an F-actin targeting module that localizes CaMKII/heterooligomers to dendritic spines. Neuron, 21, 593–606.
Svitkina, T., Verkhovsky, A., McQuade, K., & Borisy, G. (1997). Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J. Cell Biol., 139(2), 397–415.
Svitkina, T., Bulanova, E., Chaga, O., Vignjevic, D., Kojima, S., Vasiliev, J., & Borisy, G. (2003). Mechanism of filopodia initiation by reorganization of a dendritic network. J. Cell Biol., 160(3), 409–421.
Ultanir, S., Kim, J., Hall, B., Deerinck, T., Ellisman, M., & Ghosh, A. (2007). Regulation of spine morphology and spine density by NMDA receptor signaling in vivo. Proc. Natl. Acad. Sci. USA, 104(49), 19553–19558.
Van Troys, M., Huyck, L., Leyman, S., Dhaese, S., Vandekerkhove, J., & Ampe, C. (2008). Ins and outs of ADF/cofilin activity and regulation. Eur. J. Cell Biol., 87(8–9), 649–667.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below are the links to the electronic supplementary material.
MP4 5.92 MB
Rights and permissions
About this article
Cite this article
Bennett, M.R., Farnell, L. & Gibson, W.G. A Model of NMDA Receptor Control of F-actin Treadmilling in Synaptic Spines and Their Growth. Bull Math Biol 73, 2109–2131 (2011). https://doi.org/10.1007/s11538-010-9614-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-010-9614-4