Abstract
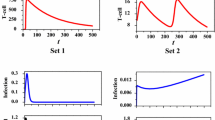
A mathematical model of the host’s immune response to HIV infection is proposed. The model represents the dynamics of 13 subsets of T cells (HIV-specific and nonspecific, healthy and infected, T4 and T8 cells), infected macrophages, neutralizing antibodies, and virus. The results of simulation are in agreement with published data regarding T4 cell concentration and viral load, and exhibit the typical features of HIV infection, i.e. double viral peaks in the acute stage, sero conversion, inverted T cell ratio, establishment of set points, steady state, and decline into AIDS. This result is achieved by taking into account thymic aging, viral and infected cell stimulation of specific immune cells, background nonspecific antigens, infected cell proliferation, viral production by infected macrophages and T cells, tropism, viral, and immune adaptation. Starting from this paradigm, changes in the parameter values simulate observed differences in individual outcomes, and predict different scenarios, which can suggest new directions in therapy. In particular, large parameter changes highlight the potentially critical role of both very vigorous and extremely damped specific immune response, and of the elimination of virus release by macrophages. Finally, the time courses of virus, antibody and T cells production and removal are systematically investigated, and a comparison of T4 and T8 cell dynamics in a healthy and in a HIV infected host is offered.
Similar content being viewed by others
References
Aquaro, S., Bagnarelli, P., Guenci, T., De Luca, A., Clement, M., Balestra, E., Caliò, R., Perno, C.F., 2002a. Long-term survival and virus production in human primary macrophages infected by human immunodeficiency virus. J. Med. Virol. 68(4), 479–488.
Aquaro, S., Caliò, R., Balzarini, J., Bellocchi, M.C., Garaci, E., Perno, C.F., 2002b. Macrophages and HIV infection: Therapeutical approaches toward this strategic virus reservoir. Antivir. Res. 55(2), 209–225.
Azzam, R., Lal, L., Goh, S.L., Kedzierska, K., Jaworowski, A., Naim, E., Cherry, C.L., Wesselingh, S.L., Mills, J., Crowe, S.M., 2006. Adverse effects of antiretroviral drugs on HIV-1-infected and uninfected human monocyte-derived macrophages. J. Acquir. Immune Defic. Syndr. 42(1), 19–28.
Bacchetti, P., Moss, A.R., 1989. Incubation period of AIDS in San Francisco. Nature 338(6212), 251–253.
Bajaria, S., Webb, G., Cloyd, M., Kirschner, D., 2002. Dynamics of naive and memory CD4+ T lymphocytes in HIV-1 disease progression. J. Acquir. Immune Defic. Syndr. 30, 41–58.
Brander, C., Suscovich, T., Lee, Y., Nguyen, P.T., O’Connor, P., Seebach, J., Jones, N.G., van Gorder, M., Crooks, E.T., Moore, P.L., Richman, D., Robinson, J., Crooks, J.A., Franti, M., Schülke, N., Binley, J.M., 2005. Characterizing anti-HIV monoclonal antibodies and immune sera by defining the mechanism of neutralization. Hum. Antib. 14(3–4), 101–113.
Chun, T.W., Engel, D., Berrey, M.M., Shea, T., Corey, L., Fauci, A.S., 1998. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 95(15), 8869–8873.
Coleman, R., Lombard, M., Sicard, R., 1992. Fundamental Immunology, 2nd edn. Wm. C Brown, Dubuque.
Collins, K.L., Chen, B.K., Kalams, S.A., Walker, B.D., Baltimore, D., 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391, 397–401.
Connor, R.I., Sheridan, K.E., Ceradini, D., Choe, S., Landau, N.R., 1997. Change in coreceptor use correlates with disease progression in HIV-1 infected individuals. J. Exp. Med. 185(4), 621–628.
Crowe, S.M., McGrath, M.S., Elbeik, T., Kirihara, J., Mills, J., 1989. Comparative assessment of antiretrovirals in human monocyte-macrophages and lymphoid cell lines acutely and chronically infected with the human immunodeficiency virus. J. Med. Virol. 29(3), 176–180.
Daar, E.S., Moudgi, L.T., Meyer, R.D., Ho, D.D., 1991. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N. Engl. J. Med. 324(14), 961–964.
Dimitrov, D.S., Xiao, X., Chabot, D., Broder, C.C., 1998. HIV coreceptors. J. Membr. Biol. 166, 75–90.
Edelstein-Keshet, L., 1988. Mathematical Models in Biology. McGraw-Hill, Boston.
Fauci, A., 2003. HIV and AIDS: 20 years of science. Nat. Med. 9(7), 839–843.
Fujiwara, M., Takiguchi, M., 2007. HIV-1-specific CTLs effectively suppress replication of HIV-1 in HIV-1-infected macrophages. Blood 109(11), 4832–4838.
Gorry, P., Churchill, M., Crowe, S.M., Cunningham, A.L., Gabuzda, D., 2005. Pathogenesis of macrophage tropic HIV-1. Curr. HIV Res. 3(1), 53–60.
Greenberg, M.E., Bronson, S., Lock, M., Neumann, M., Pavlakis, G.N., Skowronski, J., 1997. Colocalization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 16, 6964–6976.
Grossman, Z., Meier-Schellersheim, Sousa, A., Rui, M., Paul, V.W., 2002. CD4+ T-cell depletion in HIV infection: Are we closer to understanding the cause?. Nat. Med. 8(4), 319–323. Commentary.
Hare, C.B., 2006. Clinical Overview of HIV Disease. HIV InSite Knowledge Base Chapter. University of California San Francisco. http://hivinsite.ucsf.edu/InSitepage=kb-03-01-01.
Harrington, L., Janowski, K., Oliver, J., Zajac, A., Weaver, C., 2008. Memory CD4 T cells emerge from effector T-cell progenitors. Nature 452, 356–360.
Hattori, T., Komoda, H., Pahwa, S., Tateyama, M., Zhang, X., Xu, Y., Oguma, S., Tamamura, H., Fujii, N., Fukutake, K., Uchiyama, T., 1998. Decline of anti-DP107 antibody associated with clinical progression. AIDS 12(12), 1557–1559.
Hatzakis, A., Touloumi, G. et al., 2000. Effect of recent thymic emigrants on progression of HIV-1 disease. Lancet 355, 9204.
Hellerstein, M., Hanley, M.B., Cesar, S., Siler, S., Papageorgopoulos, C., Wieder, E., Schmidt, D., Hoh, R., Nesse, R., Macallan, D., Deeks, S., McCune, J.M., 1999. Directly measured kinetics in circulating T lymphocytes in normal and HIV-1-infected humans. Nat. Med. 5, 83–88.
Ho, D.D., 1996. Viral counts in HIV infection. Science 272, 1124–1125.
Ho, D.D., 1997. Dynamics of HIV-1 replication in vivo. Clin. Invest. 90(11), 2565–2567.
Ho, D.D., Neumann, U., Perelson, A., Chen, W., Leonard, J., Markowits, M., 1995. Rapid turnover of plasma virons and CD4 lymphocytes in HIV-1 infection. Nature 373, 123–126.
Igarshi, T., et al., 2001. Macrophages are the principal reservoir and sustain high virus loads in Rhesus macaques following the depletion of CD4+ T cells by a highly pathogenic SHIV: Implications for HIV-1 infections of man. Proc. Natl. Acad. Sci. 98, 658–663.
Janeway, C., Travers, P., Walport, M., 1996. Immuno Biology, 3rd edn. Elsevier Science Ltd/Garland Publishing, Amsterdam.
Janeway, C., Travers, P., Walport, M., Capra, J.D., 1999. Immuno Biology, 4th edn. Elsevier Science Ltd/Garland Publishing, Amsterdam.
Jones, L.E., Perelson, A.S., 2005. Opportunistic infection as a cause of transient viremia in chronically infected HIV patients under treatment with HAART. Bull. Math. Biol. 67, 1227–1251.
Kaneko, H., Neoh, L.P., Takeda, N., Akimoto, H., Hishikawa, T., Hashimoto, H., Hirose, S., Karaki, S., Takiguchi, M., Nakauchi, H., Kaneko, Y., Yamamoto, N., Sekigawa, I., 1997. Human immunodeficiency virus type 2 envelope glycoprotein binds to CD8 as well as to CD4 molecules on human T cells. J. Virol. 71(11), 8918–8922.
Kim, H., Perelson, A., 2006. Viral and latent reservoir persistence in HIV-1 infected patients on therapy. PLoS Comput. Biol. 2(10, e135), 1235–1247.
Kirschner, D.E., Perelson, A., 1995. A model for the immune system response to HIV: AZT treatment studies. In: Mathematical Population Dynamics: Analysis of Heterogeneity, Theory of Epidemics, vol. 1, pp. 295–310. Wuerz Publishing Ltd., Winnipeg.
Kirschner, D.E., Webb, G.F., 1996. A model for treatment strategy in the chemotherapy of AIDS. Bull. Math. Biol. 58, 367–391.
Kitchen, G., Uittenbogaart, C.H., Zack, J., 1997. Mechanism of human immunodefifiency virus type localization in CD4-negative thymocytes: Differentiation from a CD4-positive precursor allow productive infection. J. Virol. 8, 5713–5722.
Kolchinsky, P., Mirzabekov, T., Furan, M., Kiprilov, E., Cayabyab, M., Mooney, L.J., Choe, H., Sodroski, J., 1999. Adaptation of a CCR5-using, primary human immunodeficiency virus type 1 isolate for CD4 independent replication. J. Virol. 73(10), 8120–8126.
Koup, R.A., Safrit, J.T., Cao, Y., Andrews, C.A., McLeod, G., Borkowsky, W., Farthing, C., Ho, D.D., 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68(7), 4650–4655.
Kuby, J., 1997. Immunology, 3rd edn. W.H. Freeman and Co., New York.
Lee, S., Goldstein, H., Baseler, M., Adelsberger, J., Golding, H., 1997. Human immunodeficiency virus type 1 infection of mature CD3hiCD8+ thymocytes. J. Virol. 71(9), 6671–6676.
Mugwagwa, T., Witten, G., 2006. Coreceptor switching in HIV-1 subtype B and subtype C. Bull. Math. Biol. 68, 55–77.
Nishimura, Y., Igarashi, T., Haigwood, N., Sadjadpour, R., Plishka, R.J., Buckler-White, A., et al., 2002. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J. Virol. 76, 2123–2130.
Pantaleo, G., Graziosi, C., Fauci, A.S., 1993. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N. Engl. J. Med. 328, 327–335.
Perelson, A.S., Nelson, P., 1999. Mathematical analysis of HIV-1 dynamics in vivo. SIAM Rev. 41, 3–44.
Perelson, A.S., Kirschner, D.E., DeBoer, R., 1993. The dynamics of HIV infection of CD4+ T cells. Math. Biosci. 114, 81–125.
Perelson, A.S., Neumann, A.U., Markowitz, M., Leonard, J.M., Ho, D.D., 1996. HIV-1 dynamics in vivo: viron clearance rate, infected cell life-span, and viral generation time. Science 271(5255), 1582–1586.
Piatak, M., Saag, M.S., Yang, L.C., Clark, S.J., Kappes, J.C., Luk, K.C., Hahn, B.H., Shaw, G.M., Lifson, J.D., 1993. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science 259(5102), 1749–1754.
Pilcher, C.D., Price, M.A., Hoffman, I., 2004. Frequent detection of acute primary HIV infection in men in Malawi. AIDS 18, 517–524.
Poveda, E., Briz, V., Quiñones-Mateu, M., Soriano, V., 2006. HIV tropism: Diagnostic tools and implications for disease progression and treatment with entry inhibitors. AIDS 20(10), 1359–1367.
Richman, D.D., Wrin, T., Little, S.J., Petropoulos, C.J., 2003. Rapid evolution of the neutralizing antibody response to HIV type I infection. Proc. Natl. Acad. Sci. USA 100, 4144–4149.
Robertson, D.L., Hahn, B.H., Sharp, P.M., 1995. Recombination in AIDS viruses. J. Mol. Evol. 40(3), 249–259.
Roos, M.T., de Leeuw, N.A., Claessen, F.A., Huisman, H.G., Kootstra, N.A., Meyaard, L., Schellekens, P.T., Schuitemaker, H., Miedema, F., 1994. Viro-immunological studies in acute HIV-1 infection. AIDS 8(11), 1533–1538.
Rouzioux, C., 2001. Early HIV-1 DNA level predicts disease progression and death independently of HIV-RNA level and CD+4 cell count. Oral Presentation: The 1st. IAS Conference on HIV Pathogenesis and Treatment: Abstract # 23.
Sachsenberg, N., Perelson, A.S., Yerly, S., 1998. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection in healthy individuals as measured by Ki-67 antigen. J. Exp. Med. 187, 1295–1303.
Saha, K., Zhang, J., Gupta, A., Dave, R., Yimen, M., Zerhouni, B., 2001. Isolation of primary HIV-1 that target CD8+ T lymphocytes using CD8 as a receptor. Nat. Med. 7, 65–72.
Scamurra, R., Mille, D., Dahl, L., Abrahamsen, M., Kapur, V., Wahl, S., Milner, E., Janoff, E., 2000. Impact of HIV-1 infection on VH3 gene repertoire of naive human B cells. J. Immunol. 164, 5482–5491.
Seppa, N., 2000. AIDS vaccine tests well in monkeys. Sci. News 158, 260.
Shankarappa, R., Margolick, J.B., Gange, S.J., Rodrigo, A.G., Upchurch, D., Farzadegan, H., Gupta, P., Rinaldo, R., Learn, G.H., He, X., Huang, X.L., Mullins, J.I., 1999. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J. Virol. 73(12), 10489–10502.
Siliciano, J.D., Siliciano, R.F., 2005. Enhanced culture assay for detection and quantization of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol. Biol. 304, 3–15.
Stafford, M., Cao, Y., Ho, D., Corey, L., Mackall, C., Gress, R., Perelson, A., 2000. Modeling plasma virus concentration and CD4+ T cell kinetics during primary HIV infection. J. Theor. Biol. 203(3), 285–301.
Stiegler, G., Armbruster, C., Vcelar, B., Stoiber, H., Kunert, R., Michael, N.L., et al., 2001. Antiviral activity of the neutralizing antibodies 2F5 and 2G12 in asymptomatic HIV-1-infected humans: A phase I evaluation. AIDS 16, 2019–2025.
Swann, S.A., Williams, M., Story, C.M., Bobbitt, K.R., Fleis, R., Collins, K.L., 2001. HIV-1 Nef blocks transport of MHC class I molecules to the cell surface via a PI 3-kinase-dependent pathway. Virology 282, 267–277.
Swiggard, W.J., Baytop, C., Yu, J.J., Dai, J., Li, C., Schretzenmair, R., Theodosopoulos, T., O’Doherty, U., 2005. Human immunodeficiency virus type 1 can establish latent infection in resting CD4+ T cells in the absence of activating stimuli. J. Virol. 79(22), 14179–14188.
Tizard, I., 1992. Immunology, An Introduction. 3rd edn. Saunders College Publishing (Harcourt Brace Jovanovich), Fort Worth Texas.
Tran, M.M., 1999. Mathematical model of HIV in vivo. Masters Thesis, New Jersey City University [unpublished].
Tripathi, P., Agrawal, S., 2007. The role of human leukocyte antigen E and G in HIV Infection. AIDS 21(11), 1395–1404.
Tsai, W.P., Conley, S.R., Kung, H.F., Garrity, R.R., Nara, P.L., 1996. Preliminary in vitro growth cycle and transmission studies of HIV-1 in an autologous primary cell assay of blood-derived macrophages and peripheral blood mononuclear cells. Virology 226(2), 205–216.
Walker, B.D., Scadden, D.T., 2000. CTL recognition of cells latently infected with Kaposi’s sarcoma-associated herpes virus. J. Immunol. 165(4), 2077–2083.
Yamamoto, N., Ueda, M., Benson, C.E., 2007. Treatment of HIV- infected parients with Ge protein-derived macrophage activating factor (GeMAF) eradicates HIV-infection. In: Proc. 13 Int. Cong. Immunol. Italy: Medimond, Bolona, pp. 35–38.
Yamamoto, N., Ushijima, N., Koga, Y., 2009. Immunotherapy of HIV-infected patients with Gc protein-derived macrophage activating factor (GcMAF). J. Med. Virol. 81, 16–26.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wasserstein-Robbins, F. A Mathematical Model of HIV Infection: Simulating T4, T8, Macrophages, Antibody, and Virus via Specific Anti-HIV Response in the Presence of Adaptation and Tropism. Bull. Math. Biol. 72, 1208–1253 (2010). https://doi.org/10.1007/s11538-009-9488-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-009-9488-5