Abstract
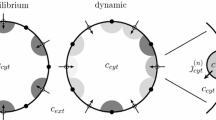
We investigate the role of heterogeneous expression of IP3R and RyR in generating diverse elementary Ca2+ signals. It has been shown empirically (Wojcikiewicz and Luo in Mol. Pharmacol. 53(4):656–662, 1998; Newton et al. in J. Biol. Chem. 269(46):28613–28619, 1994; Smedt et al. in Biochem. J. 322(Pt. 2):575–583, 1997) that tissues express various proportions of IP3 and RyR isoforms and this expression is dynamically regulated (Parrington et al. in Dev. Biol. 203(2):451–461, 1998; Fissore et al. in Biol. Reprod. 60(1):49–57, 1999; Tovey et al. in J. Cell Sci. 114(Pt. 22):3979–3989, 2001). Although many previous theoretical studies have investigated the dynamics of localized calcium release sites (Swillens et al. in Proc. Natl. Acad. Sci. U.S.A. 96(24):13750–13755, 1999; Shuai and Jung in Proc. Natl. Acad. Sci. U.S.A. 100(2):506–510, 2003a; Shuai and Jung in Phys. Rev. E, Stat. Nonlinear Soft Matter Phys. 67(3 Pt. 1):031905, 2003b; Thul and Falcke in Biophys. J. 86(5):2660–2673, 2004; DeRemigio and Smith in Cell Calcium 38(2):73–86, 2005; Nguyen et al. in Bull. Math. Biol. 67(3):393–432, 2005), so far all such studies focused on release sites consisting of identical channel types. We have extended an existing mathematical model (Nguyen et al. in Bull. Math. Biol. 67(3):393–432, 2005) to release sites with two (or more) receptor types, each with its distinct channel kinetics. Mathematically, the release site is represented by a transition probability matrix for a collection of nonidentical stochastically gating channels coupled through a shared Ca2+ domain. We demonstrate that under certain conditions a previously defined mean-field approximation of the coupling strength does not accurately reproduce the release site dynamics. We develop a novel approximation and establish that its performance in these instances is superior. We use this mathematical framework to study the effect of heterogeneity in the Ca2+-regulation of two colocalized channel types on the release site dynamics. We consider release sites consisting of channels with both Ca2+-activation and inactivation (“four-state channels”) and channels with Ca2+-activation only (“two-state channels”) and show that for the appropriate parameter values, synchronous channel openings within a release site with any proportion of two-state to four-state channels are possible, however, the larger the proportion of two-state channels, the more sensitive the dynamics are to the exact spatial positioning of the channels and the distance between channels. Specifically, the clustering of even a small number of two-state channels interferes with puff/spark termination and increases puff durations or leads to a tonic response.
Similar content being viewed by others
References
Berridge, M., 1993. Inositol trisphosphate and Ca2+ signaling. Nature 361(6410), 315–325.
Berridge, M., 1997. Elementary and global aspects of Ca2+ signalling. J. Physiol. (Lond.) 499(Pt. 2), 291–306.
Berridge, M., Dupont, G., 1994. Spatial and temporal signalling by calcium. Curr. Opin. Cell. Biol. 6(2), 267–274.
Carlson, G., Slawecki, M., Lancaster, E., Keller, A., 1997. Distribution and activation of intracellular Ca2+ stores in cultured olfactory bulb neurons. J. Neurophysiol. 78(4), 2176–2185.
Dawson, A., Lea, E., Irvine, R., 2003. Kinetic model of the inositol trisphosphate receptor that shows both steady-state and quantal patterns of Ca2+ release from intracellular stores. Biochem. J. 370(Pt. 2), 621–629.
DeRemigio, H., Smith, G., 2005. The dynamics of stochastic attrition viewed as an absorption time on a terminating Markov chain. Cell Calcium 38(2), 73–86.
DeRemigio, H., Groff, J.R., Smith, G.D., 2008. Calcium release site ultrastructure and the dynamics of puffs and sparks. Math. Med. Biol. 25(1), 65–85.
De Young, G., Keizer, J., 1992. A single-pool inositol 1, 4, 5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc. Natl. Acad. Sci. U.S.A. 89(20), 9895–9899.
Domeier, T., Zima, A., Maxwell, J., Huke, S., Mignery, G., Blatter, L., 2007. Ip3 receptor-dependent Ca2+ release modulates excitation-contraction coupling in rabbit ventricular myocytes. Am. J. Physiol., Heart Circ. Physiol.
Dummit, D.S., Foote, R.M., 1999. Abstract Algebra. Wiley, New York.
Falcke, M., 2004. Reading the patterns in living cells—the physics of Ca2+ signaling. Adv. Phys. 53(3), 255–440.
Fill, M., Zahradnikova, A., Villalba-Galea, C., Zahradnik, I., Escobar, A., Gyorke, S., 2000. Ryanodine receptor adaptation. J. Gen. Physiol. 116(6), 873–882.
Fissore, R., Longo, F., Anderson, E., Parys, J., Ducibella, T., 1999. Differential distribution of inositol trisphosphate receptor isoforms in mouse oocytes. Biol. Reprod. 60(1), 49–57.
Groff, J.R., Smith, G.D., 2008a. Ryanodine receptor allosteric coupling and the dynamics of calcium sparks. Biophys. J. 95(1), 135–154.
Groff, J.R., Smith, G.D., 2008b. Calcium-dependent inactivation and the dynamics of calcium puffs and sparks. J. Theor. Biol. 253(3), 483–499.
Gyorke, S., Fill, M., 1993. Ryanodine receptor adaptation: control mechanism of Ca2+-induced Ca2+ release in heart. Science 260(5109), 807–809.
Haak, L., Song, L., Molinski, T., Pessah, I., Cheng, H., Russell, J., 2001. Sparks and puffs in oligodendrocyte progenitors: cross talk between ryanodine receptors and inositol trisphosphate receptors. J. Neurosci. 21(11), 3860–3870.
Hattori, M., Suzuki, A., Higo, T., Miyauchi, H., Michikawa, T., Nakamura, T., Inoue, T., Mikoshiba, K., 2004. Distinct roles of inositol 1, 4, 5-trisphosphate receptor types 1 and 3 in Ca2+ signaling. J. Biol. Chem. 279(12), 11967–11975.
Hill, T., 1977. Free Energy Transduction in Biology: The Steady-State Kinetic and Thermodynamic Formalism. Academic Press, New York.
Janiak, R., Wilson, S., Montague, S., Hume, J., 2001. Heterogeneity of Ca2+ stores and elementary release events in canine pulmonary arterial smooth muscle cells. Am. J. Physiol., Cell. Physiol. 280(1), C22–C33.
Kajimoto, K., Daikoku, T., Yamazaki, N., Terada, H., Shinohara, Y., 2003. Expression profiles of three isoforms of inositol 1, 4, 5-trisphosphate receptor in brown adipose tissue of the rat. Biochem. Pharmacol. 65(6), 995–998.
Keizer, J., De Young, G., 1994. Simplification of a realistic model of IP3-induced Ca2+ oscillations. J. Theor. Biol. 166, 431–442.
Koizumi, S., Lipp, P., Berridge, M., Bootman, M., 1999. Regulation of ryanodine receptor opening by lumenal Ca2+ underlies quantal Ca2+ release in pc12 cells. J. Biol. Chem. 274(47), 33327–33333.
Laver, D., 2005. Coupled calcium release channels and their regulation by luminal and cytosolic ions. Eur. Biophys. J. 34(5), 359–368.
Li, Y., Rinzel, J., 1994. Equations for IP3R-mediated [Ca2+] i oscillations derived from a detailed kinetic model: a Hodgkin–Huxley like formalism. J. Theor. Biol. 166(4), 461–473.
Lipp, P., Laine, M., Tovey, S., Burrell, K., Berridge, M., Li, W., Bootman, M., 2000. Functional Insp3 receptors that may modulate excitation-contraction coupling in the heart. Curr. Biol. 10(15), 939–942.
Mackenzie, L., Bootman, M., Laine, M., Berridge, M., Thuring, J., Holmes, A., Li, W., Lipp, P., 2002. The role of inositol 1, 4, 5-trisphosphate receptors in Ca2+ signalling and the generation of arrhythmias in rat atrial myocytes. J. Physiol. 541(Pt. 2), 395–409.
MacKrill, J., 1999. Protein-protein interactions in intracellular Ca2+-release channel function. Biochem. J. 337(Pt. 3), 345–361.
Mazzag, B., Tignanelli, C., Smith, G., 2005. The effect of residual Ca2+ on the stochastic gating of Ca2+-regulated Ca2+ channel models. J. Theor. Biol. 235(1), 121–150.
Miyakawa, T., Maeda, A., Yamazawa, T., Hirose, K., Kurosaki, T., Iino, M., 1999. Encoding of Ca2+ signals by differential expression of Ip3 receptor subtypes. EMBO J. 18(5), 1303–1308.
Moraru, I., Kaftan, E., Ehrlich, B., Watras, J., 1999. Regulation of type 1 inositol 1, 4, 5-trisphosphate-gated Ca2+ channels by IP3 and Ca2+: simulation of single channel kinetics based on ligand binding and electrophysiological analysis. J. Gen. Physiol. 113(6), 837–849.
Moschella, M., Marks, A., 1993. Inositol 1, 4, 5-trisphosphate receptor expression in cardiac myocytes. J. Cell Biol. 120(5), 1137–1146.
Naraghi, M., Neher, E., 1997. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a Ca2+ channel. J. Neurosci. 17, 6961.
Neher, E., 1986. Concentration profiles of intracellular Ca2+ in the presence of diffusible chelator. Exp. Brain Res. 14, 80–96.
Newton, C., Mignery, G., Sudhof, T., 1994. Co-expression in vertebrate tissues and cell lines of multiple inositol 1, 4, 5-trisphosphate (Insp3) receptors with distinct affinities for Insp3. J. Biol. Chem. 269(46), 28613–28619.
Nguyen, V., Mathias, R., Smith, G., 2005. A stochastic automata network descriptor for Markov chain models of instantaneously coupled intracellular Ca2+ channels. Bull. Math. Biol. 67(3), 393–432.
Parrington, J., Brind, S., Smedt, H.D., Gangeswaran, R., Lai, F., Wojcikiewicz, R., Carroll, J., 1998. Expression of inositol 1, 4, 5-trisphosphate receptors in mouse oocytes and early embryos: the type i isoform is upregulated in oocytes and downregulated after fertilization. Dev. Biol. 203(2), 451–461.
Roderick, H., Bootman, M., 2003. Bi-directional signalling from the Insp3 receptor: regulation by calcium and accessory factors. Biochem. Soc. Trans. 31(Pt. 5), 950–953.
Rossi, D., Simeoni, I., Micheli, M., Bootman, M., Lipp, P., Allen, P., Sorrentino, V., 2002. Ryr1 and Ryr3 isoforms provide distinct intracellular Ca2+ signals in HEK 293 cells. J. Cell Sci. 115(Pt. 12), 2497–2504.
Shuai, J., Jung, P., 2003a. Optimal ion channel clustering for intracellular calcium signaling. Proc. Natl. Acad. Sci. U.S.A. 100(2), 506–510.
Shuai, J., Jung, P., 2003b. Selection of intracellular calcium patterns in a model with clustered Ca2+ release channels. Phys. Rev. E, Stat. Nonlinear Soft Matter Phys. 67(3 Pt. 1), 031905.
Shuai, J., Pearson, J., Foskett, J., Mak, D.D., Parker, I., 2007. A kinetic model of single and clustered Ip3 receptors in the absence of Ca2+ feedback. Biophys. J. 93, 1151–1162.
Smedt, H.D., Missiaen, L., Parys, J., Bootman, M., Mertens, L., Bosch, L.V.D., Casteels, R., 1994. Determination of relative amounts of inositol trisphosphate receptor mrna isoforms by ratio polymerase chain reaction. J. Biol. Chem. 269(34), 21691–21698.
Smedt, H.D., Missiaen, L., Parys, J., Henning, R., Sienaert, I., Vanlingen, S., Gijsens, A., Himpens, B., Casteels, R., 1997. Isoform diversity of the inositol trisphosphate receptor in cell types of mouse origin. Biochem. J. 322(Pt. 2), 575–583.
Smith, G., Dai, L., Muira, R., Sherman, A., 2001. Asymptotic analysis of equations for the buffered diffusion of intracellular Ca2+. SIAM J. Appl. Math. 61(5), 1816–1838.
Sneyd, J., Dufour, J., 2002. A dynamic model of the type-2 inositol trisphosphate receptor. Proc. Natl. Acad. Sci. U.S.A. 99(4), 2398–2403.
Solomon, F., 1987. Probability and Stochastic Processes. Prentice-Hall, Englewood Cliffs.
Stern, M., Song, L., Cheng, H., Sham, J., Yang, H., Boheler, K., Rios, E., 1999. Local control models of cardiac excitation-contraction coupling: a possible role for allosteric interactions between ryanodine receptors. J. Gen. Physiol. 113(3), 469–489.
Stewart, W., 1994. Introduction to the Numerical Solution of Markov Chains. Princeton University Press, Princeton.
Swatton, J., Taylor, C., 2002. Fast biphasic regulation of type 3 inositol trisphosphate receptors by cytosolic calcium. J. Biol. Chem. 277(20), 17571–17579.
Swillens, S., Dupont, G., Combettes, L., Champeil, P., 1999. From Ca2+ blips to Ca2+ puffs: theoretical analysis of the requirements for interchannel communication. Proc. Natl. Acad. Sci. U.S.A. 96(24), 13750–13755.
Tang, Y., Othmer, H., 1994. A model of calcium dynamics in cardiac myocytes based on the kinetics of ryanodine-sensitive calcium channels. Biophys. J. 67(6), 2223–2235.
Tasker, P., Michelangeli, F., Nixon, G., 1999. Expression and distribution of the type 1 and type 3 inositol 1,4, 5-trisphosphate receptor in developing vascular smooth muscle. Circ. Res. 84(5), 536–542.
Thrower, E., Hagar, R., Ehrlich, B., 2001. Regulation of Ins(1, 4, 5)p3 receptor isoforms by endogenous modulators. Trends Pharmacol. Sci. 22(11), 580–586.
Thul, R., Falcke, M., 2004. Release currents of IP3 receptor channel clusters and concentration profiles. Biophys. J. 86(5), 2660–2673.
Tovey, S., de Smet, P., Lipp, P., Thomas, D., Young, K., Missiaen, L., Smedt, H.D., Parys, J., Berridge, M., Thuring, J., Holmes, A., Bootman, M., 2001. Calcium puffs are generic Insp(3)-activated elementary calcium signals and are downregulated by prolonged hormonal stimulation to inhibit cellular calcium responses. J. Cell Sci. 114(Pt. 22), 3979–3989.
Tu, H., Nosyreva, E., Miyakawa, T., Wang, Z., Mizushima, A., Iino, M., Bezprozvanny, I., 2003. Functional and biochemical analysis of the type 1 inositol (1, 4, 5)-trisphosphate receptor calcium sensor. Biophys. J. 85(1), 290–299.
Tu, H., Wang, Z., Bezprozvanny, I., 2005a. Modulation of mammalian inositol 1, 4, 5-trisphosphate receptor isoforms by calcium: a role of calcium sensor region. Biophys. J. 88(2), 1056–1069.
Tu, H., Wang, Z., Nosyreva, E., Smedt, H.D., Bezprozvanny, I., 2005b. Functional characterization of mammalian inositol 1, 4, 5-trisphosphate receptor isoforms. Biophys. J. 88(2), 1046–1055.
Vermassen, E., Parys, J., Mauger, J., 2004. Subcellular distribution of the inositol 1, 4, 5-trisphosphate receptors: functional relevance and molecular determinants. Biol. Cell 96(1), 3–17.
Williams, G., Huertas, M., Sobie, E., Jafri, M., Smith, G., 2007. A probability density approach to modeling local control of calcium-induced calcium release in cardiac myocytes. Biophys. J. 92(7), 2311–2328.
Wojcikiewicz, R., 1995. Type i, ii, and iii inositol 1, 4, 5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J. Biol. Chem. 270(19), 11678–11683.
Wojcikiewicz, R., Luo, S., 1998. Differences among type i, ii, and iii inositol-1, 4, 5-trisphosphate receptors in ligand-binding affinity influence the sensitivity of calcium stores to inositol-1, 4, 5-trisphosphate. Mol. Pharmacol. 53(4), 656–662.
Woodcock, E., Matkovich, S., 2005. Ins(1, 4, 5)p3 receptors and inositol phosphates in the heart-evolutionary artefacts or active signal transducers? Pharmacol. Ther. 107(2), 240–251.
Zahradnikova, A., Zahradnik, I., Gyorke, I., Gyorke, S., 1999. Rapid activation of the cardiac ryanodine receptor by submillisecond calcium stimuli. J. Gen. Physiol. 114(6), 787–798.
Zima, A., Blatter, L., 2004. Inositol-1, 4, 5-trisphosphate-dependent Ca2+ signalling in cat atrial excitation-contraction coupling and arrhythmias. J. Physiol. 555(Pt. 3), 607–615.
Author information
Authors and Affiliations
Corresponding author
Additional information
The first two authors contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Cooper, Z., Greenwood, M. & Mazzag, B. A Computational Analysis of Localized Ca2+-Dynamics Generated by Heterogeneous Release Sites. Bull. Math. Biol. 71, 1543–1579 (2009). https://doi.org/10.1007/s11538-009-9413-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-009-9413-y