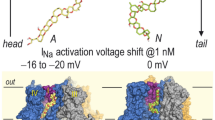
Abstract
Ciguatoxins, a group of virulent marine toxins, are bound to "site 5" inside voltage-dependent sodium channels and alter their kinetics dramatically; this is probably responsible for clinical symptoms of ciguatoxin poisoning or ciguatera. Although ciguatoxins are known to affect the voltage dependency of both activation and inactivation kinetics, site 5 has not been functionally clarified. This study, therefore, targeted putative voltage sensors as a receptor for ciguatoxins. We constructed mutants, in which 1 of 23 basic residues was substituted with glutamine in the S4 segment for each of four domains. We then examined the effects of a synthetic ciguatoxin congener CTX3C on these mutants. Notably, the suppressive effect of CTX3C on the sodium current (I Na) amplitude of domain 2 mutants, which carried a mutation in the basic S4 residue of domain 2, was either reduced or eliminated. Kinetic analyses of domain 2 mutants in comparison with those of the wild type and other mutants revealed that the negative shift of the steady-state inactivation curve (V 1/2inactΔ) was significantly decreased. The resistance of domain 2 mutants to CTX3C in terms of changes in V 1/2inact is suggested to be closely related to resistance to the suppressive effect of CTX3C on the I Na amplitude. This is the first report to demonstrate the existence of a functional relationship between a ciguatoxin congener and voltage sensor segments in domain 2 of the α-subunit of voltage-dependent sodium channels, although further studies are needed to locate a ciguatoxin receptor.




Similar content being viewed by others
References
Bagnis R, Chanteau S, Chungue E, Hurtel JM, Yasumoto T, Inoue A (1980) Origins of ciguatera fish poisoning: a new dinoflagellate, Gambierdiscus toxicus Adachi and Fukuyo, definitively involved as a causal agent. Toxicon 18:199–208
Yasumoto T (2001) The chemistry and biological function of natural marine toxins. Chem Rec 1:228–242
Watters MR (1995) Organic neurotoxins in seafoods. Clin Neurol Neurosurg 97:119–124
Oshiro N, Yogi K, Asato S, Sasaki T, Tamanaha K, Hirama M, Yasumoto T, Inafuku Y (2010) Ciguatera incidence and fish toxicity in Okinawa, Japan. Toxicon 56:656–661
Hamilton B, Whittle N, Shaw G, Eaglesham G, Moore MR, Lewis RJ (2010) Human fatality associated with Pacific ciguatoxin contaminated fish. Toxicon 56:668–673
Murata M, Legrand AM, Ishibashi Y, Fukui M, Yasumoto T (1990) Structures and configurations of ciguatoxin from the moray eel Gymnothorax javanicus and its likely precursor from the dinoflagellate Gambierdiscus toxicus. J Am Chem Soc 112:4380–4386
Satake M, Murata M, Yasumoto T (1993) The structure of CTX3C, a ciguatoxin congener isolated from cultured Gambierdiscus toxicus. Tetrahedron Lett 34:1975–1978
Bidard JN, Vijverberg HP, Frelin C, Chungue E, Legrand AM, Bagnis R, Lazdunski M (1984) Ciguatoxin is a novel type of Na+ channel toxin. J Biol Chem 259:8353–8357
Hattori H, Hirata Y, Hamajima M, Kaneko R, Ito K, Ishii A, Suzuki O, Seno H (2009) Simultaneous analysis of aconitine, mesaconitine, hypaconitine, and jesaconitine in whole blood by LC–MS–MS using a new polymer column. Forensic Toxicol 27:7–11
Hogg RC, Lewis RJ, Adams DJ (2002) Ciguatoxin-induced oscillations in membrane potential and action potential firing in rat parasympathetic neurons. Eur J Neurosci 16:242–248
Seino A, Kobayashi M, Momose K, Yasumoto T, Ohizumi Y (1988) The mode of inotropic action of ciguatoxin on guinea-pig cardiac muscle. Br J Pharmacol 95:876–882
Hidalgo J, Liberona JL, Molg J, Jaimovich E (2002) Pacific ciguatoxin-1b effect over Na+ and K+ currents, inositol 1,4,5-triphosphate content and intracellular Ca2+ signals in cultured rat myotubes. Br J Pharmacol 137:1055–1062
Hirama M, Oishi T, Uehara H, Inoue M, Maruyama M, Oguri H, Satake M (2001) Total synthesis of ciguatoxin CTX3C. Science 294:1904–1907
Yamaoka K, Inoue M, Miyahara H, Miyazaki K, Hirama M (2004) A quantitative and comparative study of the effects of a synthetic ciguatoxin CTX3C on the kinetic properties of voltage-dependent sodium channels. Br J Pharmacol 142:879–889
Yamaoka K, Inoue M, Miyazaki K, Hirama M, Kondo C, Kinoshita E, Miyoshi H, Seyama I (2009) Synthetic ciguatoxins selectively activate Nav1.8-derived chimeric sodium channels expressed in HEK293 cells. J Biol Chem 284:7597–7605
Trainer VL, Baden DG, Catterall WA (1994) Identification of peptide components of the brevetoxin receptor site of rat brain sodium channels. J Biol Chem 269:19904–19909
Lombet A, Bidard JN, Lazdunski M (1987) Ciguatoxin and brevetoxins share a common receptor site on the neuronal voltage-dependent Na+ channel. FEBS Lett 219:355–359
Jiang Y, Ruta V, Chen J, Lee A, MacKinnon R (2003) The principle of gating charge movement in a voltage-dependent K+ channel. Nature 423:42–48
Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R (2003) X-Ray structure of a voltage-dependent K+ channel. Nature 423:33–41
Kimura T, Yamaoka K, Kinoshita E, Maejima H, Yuki T, Yakehiro M, Seyama I (2001) Novel site on sodium channel alpha-subunit responsible for the differential sensitivity of grayanotoxin in skeletal and cardiac muscle. Mol Pharmacol 60:865–872
Kimura T, Kinoshita E, Yamaoka K, Yuki T, Yakehiro M, Seyama I (2000) On site of action of grayanotoxin in domain 4 segment 6 of rat skeletal muscle sodium channel. FEBS Lett 465:18–22
Ishii H, Kinoshita E, Kimura T, Yakehiro M, Yamaoka K, Imoto K, Mori Y, Seyama I (1999) Point-mutations related to the loss of batrachotoxin binding abolish the grayanotoxin effect in Na+ channel isoforms. Jpn J Physiol 49:457–461
Moriyoshi K, Richards LJ, Akazawa C, O’Leary DD, Nakanishi S (1996) Labeling neural cells using adenoviral gene transfer of membrane-targeted GFP. Neuron 16:255–260
Isom LL, De Jongh KS, Patton DE, Reber BF, Offord J, Charbonneau H, Walsh K, Goldin AL, Catterall WA (1992) Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science 256:839–842
Inoue M, Miyazaki K, Uehara H, Maruyama M, Hirama M (2004) First- and second-generation total synthesis of ciguatoxin CTX3C. Proc Natl Acad Sci USA 101:12013–12018
Inoue M, Miyazaki K, Ishihara Y, Tatami A, Ohnuma Y, Kawada Y, Komano K, Yamashita S, Lee N, Hirama M (2006) Total synthesis of ciguatoxin and 51-hydroxy CTX3C. J Am Chem Soc 128:9352–9354
Yamashita S, Ishihara Y, Morita H, Uchiyama J, Takeuchi K, Inoue M, Hirama M (2011) Stereoselective 6-exo radical cyclization using cis-vinyl sulfoxide: practical total synthesis of CTX3C. J Nat Prod 74:357–364
Catterall WA (1976) Purification of a toxic protein from scorpion venom which activates the action potential Na+ ionophore. J Biol Chem 251:5528–5536
Cahalan MD (1975) Modification of sodium channel gating in frog myelinated nerve fibres by Centruroides sculpturatus scorpion venom. J Physiol 244:511–534
Cestèle S, Qu Y, Rogers JC, Rochat H, Scheuer T, Catterall WA (1998) Voltage sensor-trapping: enhanced activation of sodium channels by beta-scorpion toxin bound to the S3–S4 loop in domain II. Neuron 21:919–931
Mitrovic N, George AL Jr, Horn R (1998) Independent versus coupled inactivation in sodium channels. Role of the domain 2 S4 segment. J Gen Physiol 111:451–462
Kinoshita E, Maejima H, Yamaoka K, Konno K, Kawai N, Shimizu E, Yokote S, Nakayama H, Seyama I (2001) Novel wasp toxin discriminates between neuronal and cardiac sodium channels. Mol Pharmacol 59:1457–1463
Kuhn FJ, Greeff NG (1999) Movement of voltage sensor S4 in domain 4 is tightly coupled to sodium channel fast inactivation and gating charge immobilization. J Gen Physiol 114:167–183
Sheets MF, Kyle JW, Kallen RG, Hanck DA (1999) The Na channel voltage sensor associated with inactivation is localized to the external charged residues of domain IV, S4. Biophys J 77:747–757
Stuhmer W, Conti F, Suzuki H, Wang XD, Noda M, Yahagi N, Kubo H, Numa S (1989) Structural parts involved in activation and inactivation of the sodium channel. Nature 339:597–603
Acknowledgments
This work was supported by a Grant-in-Aid for Specially Promoted Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. We thank Ms. C. Kondo for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Part of this study was presented at the 29th Annual Meeting of Japanese Association of Forensic Toxicology, Tokyo, as part of the symposium “Research and Analysis on Marine Neurotoxins at the Forefront”.
Rights and permissions
About this article
Cite this article
Yamaoka, K., Inoue, M. & Hirama, M. A study on mechanisms of toxic actions of ciguatoxins: existence of functional relationship between CTX3C and charged residues of voltage sensors in Nav1.4 sodium channel. Forensic Toxicol 29, 125–131 (2011). https://doi.org/10.1007/s11419-011-0113-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-011-0113-6