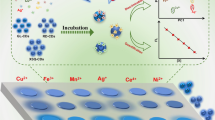
Abstract
The need for the sensing of environmental pollutants cannot be overemphasized in the twenty-first century. Herein, a sensor has been developed for the sensitive and selective detection of copper (Cu2+), lead (Pb2+) and cadmium (Cd2+) as major heavy metals polluting water environment. A screen-printed carbon electrode (SPCE) modified by fluorescent carbon dots (CDs) and gold nanoparticles (AuNPs) was successfully fabricated for sensing Cu2+, Pb2+ and Cd2+. Differential pulse voltammetry (DPV) and cyclic voltammetry (CV) were deployed for the analysis of ternary analytes. CV was set at a potential range of − 0.8 to + 0.2 V at a scan rate of 100 mV/s, and DPV at a potential range of − 0.8 to + 0.1 V, scan rate of 50 mV/s, pulse rate of 0.2 V and pulse width of 50 ms. DPV technique was applied through the modified electrode for sensitive and selective determination of Cu2+, Pb2+ and Cd2+ at a concentration range of 0.01 to 0.27 ppm for Cu2+, Pb2+ and Cd2+. Tolerance for the highest possible concentration of foreign substances such as Mg2+, K+, Na+, NO3−, and SO42− was observed with a relative error less than ± 3%. The sensitivity of the modified electrode was at 0.17, 0.42 and 0.18 ppm for Cd2+, Pb2+ and Cu2+, respectively, while the limits of detection (LOD) achieved for cadmium, lead and copper were 0.0028, 0.0042 and 0.014 ppm, respectively. The quality of the modified electrode for sensing Cu2+, Pb2+ and Cd2+ at trace levels is in accordance with the World Health Organization (WHO) and Environmental Protection Agency (EPA) water regulation standard. The modified SPCE provides a cost-effective, dependable and stable means of detecting heavy metal ions (Cu2+, Pb2+ and Cd2+) in an aqueous solution.

.






Similar content being viewed by others
References
Akajionu BC, Reza H, Nor AY et al (2017) Fabrication of reduced graphene oxide-magnetic nanocomposite (rGOFe3O4) as an electrochemical sensor for trace determination of As(III) in water resources. J Elctroanal Chem 796:33–42
Amin N, Hussain A, Alamzeb S et al (2013) Accumulation of heavy metals in edible parts of vegetables irrigated with waste water and their daily intake to adults and children, District Mardan. Pkt Fd Chem 136:1515–1523
An B, Zhao D (2012) Immobilization of As(III) in soil and groundwater using a new class of polysaccharide stabilized Fe–Mn oxide nanopartiparticles. J Hazrd Mater 211:332–341
An B, Fu Z, Xiong Z et al (2010) Synthesis and characterization of a new class of polymeric ligand exchangers for selective removal of arsenate from drinking water. React Funct Polym 70:497–507
An B, Liang Q, Zhao D (2011) Removal of arsenic (V) from spent ion exchange brine using a new class of starch-bridged magnetite nanoparticles. Water Res 45:1961–1972
Asghari A (2008) Simultaneous determination of trace amounts of lead and zinc by adsorptive cathodic stripping voltammetry. My J Anal Sci 12:410–418
Aziz MA, Sohail M, Oyama M et al (2015) Electrochemical investigation of metal oxide conducting electrodes for direct detection of sulfide. Electroanalysis 27:1268–1275
Baghayeria M, Amirhassan A, Behrooz M et al (2018) A simple approach for simultaneous detection of cadmium (II) and lead(II) based on glutathione coated magnetic nanoparticles as a highly selective electrochemical probe. Sens and Actuators B Chem 273:1442–1450
Bridewell VL, Karwacki CJ, Kamat PV (2016) Electrocatalytic sensing with reduced graphene oxide: electron shuttling between redox couples anchored on a 2-D surface. ACS Sens 1:1203–1207
Chaiyoa S, Amara A, Weena S et al (2016) High sensitivity and specificity simultaneous determination of lead, cadmium and copper using μPAD with dual electrochemical and colorimetric detection. Sens Actuators B Chem 233:540–549
Devasenathipathy R, Mani V, Chen SM et al (2014) Electrodeposition of gold nanoparticles on a pectin scaffold and its electrocatalytic application in the selective determination of dopamine. RSC Adv 4:55900–55907
Fatima ES, Aicha O, Mama ER (2017) Electrochemical detection of lead (II) at bismuth/poly(1,8-diaminonaphthalene) modified carbon paste electrode. Arab J Chem 10:596–603
Gayen P, Chaplin BP (2016) Selective electrochemical detection of ciprofloxacin with a porous nafion/multiwalled carbon nanotube composite film electrode. ACS Appl Mater Interfaces 8:1615–1626
Han B, Zhang M, Zhao D, Feng Y (2015) Degradation of aqueous and soil-sorbed estradiol using a new class of stabilized manganese oxide nanoparticles. Water Res 70:288–299
Hayat A, Marty JL (2014) Disposable screen printed electrochemical sensors: tools for environmental monitoring. Sens 14:10432–10453
He F, Zhao D (2005) Preparation and characterization of a new class of starch-stabilized bimetallic nanoparticles for degradation of chlorinated hydrocarbons in water. Environ Sci Technol 39:3314–3320
He F, Zhao D (2007) Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers. Environ Sci Technol 41:6216–6221
He F, Zhao D, Paul C (2010) Yield assessment of carboxymethyl cellulose stabilized iron nanoparticles for in situ destruction of chlorinated solvents in source zones. Water Res 44:2360–2370
Kakhki S, Barsan MM, Shams E et al (2013) New redox and conducting polymer modified electrodes for cholesterol biosensing. Anal Methods 5:1199–1204
Kristie CA, Clarissa ET, Royce NDS et al (2010) Individual and simultaneous determination of lead, cadmium, and zinc by anodic stripping voltammetry at a bismuth bulk electrode. Talanta 82:675–680
Kumar R, Bhuvana T, Sharma A (2017) Nickel tungstate–graphene nanocomposite for simultaneous electrochemical detection of heavy metal ions with application to complex aqueous media. RSC Adv 7:42146–42158
Lei L, Ke Z, Yumin W (2019) A simple strategy for the detection of Cu(II), Cd(II) and Pb(II) in water by a voltammetric sensor on a TC4A modified electrode. New J Chem 43:1544–1550
Li M, Li YT, Li DW, Long YT (2012) Recent developments and applications of screen-printed electrodes in environmental assays—a review. Anal Chim Acta 734:31–44
Liu W (2017) Preparation of a zinc oxide-reduced graphene oxide nanocomposite for the determination of cadmium (II), lead (II), copper (II), and mercury (II) in water. Int J Electrochem Sci 12:5392–5403
Liu L, Zhang K, Wei Y (2019) A simple strategy for the detection of Cu (ii), Cd (ii) and Pb (ii) in water by a voltammetric sensor on a TC4A modified electrode. New J Chem 43:1544–1550
Meng Y, Kong D, Wang R et al (2017) Electrochemical co-detection of heavy metals in Astragalus membranaceus by anodic stripping voltammetry. Int J Electrochem Sci 12:8106–8119
Müller NC, Nowack B (2010). Nano zero valent iron—the solution for water and soil remediation. 1–34: Report of the Observatory NANO
Musa YP, Abidin ZZ, Suraya AR et al (2019a) Synthesis and characterization of fluorescent carbon dots from tapioca. ChemistrySelect 4:1–8
Musa YP, Abidin ZZ, Suraya AR et al (2019b) Sustainable synthesis processes for carbon dots through response surface methodology and artificial neural network. Processes 7:704–723
Noman E, Al-Gheethi A, Talip BA et al (2019) Inactivating pathogenic bacteria in greywater by biosynthesized Cu/Zn nanoparticles from secondary metabolite of Aspergillus iizukae; optimization, mechanism and techno economic analysis. PLoS One 14:1–21
Rudra K, Thiruvelu B, Ashutosh S (2017) Nickel tungstate–graphene nanocomposite for simultaneous electrochemical detection of heavy metal ions with application to complex aqueous media. RSC Adv 7:42146
Salih FE, Ouarzane A, El Rhazi M (2017) Electrochemical detection of lead (II) at bismuth/poly (1, 8-diaminonaphthalene) modified carbon paste electrode. Arab J Chem 10:596–603
Simpson A, Pandey RR, Charles CC et al (2018) Fabrication characterization and potential applications of carbon nanoparticles in the detection of heavy metal ions in aqueous media. Carbon 127:122–130
Singh RP, Agrawal M (2010) Variations in heavy metal accumulation, growth and yield of rice plants grown at different sewage sludge amendment rates. Ecotoxicol Environ Saf 73:632–641
Syahraini SS, Wan FWK, Zainiharyati MZ et al (2018) Detection of cadmium by using ionic liquid cellulose based thin layer. Intl J Eng'g Tech 7:197–203
Tratnyek PG, Salter-Blanc A, Nurmi J et al (2011). Reactivity of zerovalent metals in aquatic media: effects of organic surface coatings. Richland, WA (US): Pacific Northwest National Laboratory (PNNL), Richland, WA (US), Environmental Molecular Sciences Laboratory (EMSL)
Vara H, Collazos-Castro JE (2015) Biofunctionalized conducting polymer/carbon microfiber electrodes for ultrasensitive neural recordings. ACS Appl Mater Interfaces 7:27016–27026
Wang Z, Xiao X, Zou T et al (2019) Citric acid capped CdS quantum dots for fluorescence detection of copper ions (II) in aqueous solution. Nanomaterials 9:32–49
Wen L, Shuting T, Xiao Z et al (2015) Application of stabilized nanoparticles for in situ remediation of metal-contaminated soil and groundwater: a critical review. Water Pollutn 1:280–291
Xiaoli L, Zhiyong Z, Ting S et al (2018) Graphene/gold nanoparticle composite-based paper sensor for electrochemical detection of hydrogen peroxide. Fullerenes Nanotubes Carb Nanostrctres 67:1536–4046
Xiong Z, He F, Zhao D, Barnett MO (2009) Immobilization of mercury in sediment using stabilized iron sulfide nanoparticles. Water Res 43:5171–5179
Xu Y, Zhao D (2007) Reductive immobilization of chromate in water and soil using stabilized iron nanoparticles. Water Res 41:2101–2108
Yu JY, Li W (2018) Gold nanoparticles/graphene oxide composite for electrochemical sensing of hydroxylamine and hydrogen peroxide. Fullerenes Nanotubes Carbon Nanostructrs 26:195–204
Zhao XH, Kong RM, Zhang XB, Meng HM, Liu WN, Tan W, Shen GL, Yu RQ (2011) Graphene-DNAzyme based biosensor for amplified fluorescence-on detection of pb2+ with a high selectivity. Anal Chem 83:5062–5066
Zuzana K, Tomas S, Pavlina A et al (2017) Determination of zinc, cadmium, lead, copper and silver using a carbon paste electrode and a screen printed electrode modified with chromium (III) oxide. Sens 17:1–14
Funding
This research is funded by the Universiti Putra Malaysia, Malaysia, under the GP-IPS/2017/9556800 grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Bingcai Pan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pudza, M.Y., Abidin, Z.Z., Abdul-Rashid, S. et al. Selective and simultaneous detection of cadmium, lead and copper by tapioca-derived carbon dot–modified electrode. Environ Sci Pollut Res 27, 13315–13324 (2020). https://doi.org/10.1007/s11356-020-07695-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07695-7