Abstract
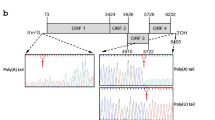
Two isolates of Pepper mild mottle virus (PMMoV) were selected from a nationwide survey of pepper fields in South Korea in 2014 and 2015, in which Cucumber mosaic virus was also detected; the two PMMoV isolates, Sangcheong 47 (S-47, KX399390) and Jeongsong 76 (J-76, KX399389), share ~99% nucleotide and amino acid identity and are closely related to Japanese and Chinese isolates at the nucleotide level. Amino acid sequence comparisons revealed 99.73, 99.81, 98.44, and 100% identity in the ORF1, ORF2, MP, and CP, respectively, between S-47 and J-76. In addition, we generated infectious clones of S-47 and J-76, and T7 promoter driven transcripts of each inoculated to Nicotiana benthamiana produced very severe symptoms, whereas only mild symptoms developed in Capsicum annuum. Gene silencing suppressor function of 126 kDa and cytoskeleton-connected plasmodesmata localization of movement protein of S-47 and J-76 showed no difference between isolates, whereas 126 kDa of J-76 clearly formed intracellular aggregates not observed with S-47 126 kDa protein. Differences between these isolates in 126/183 kDa-related functions including subcellular localization suggest that differential interactions with host proteins may affect symptom development in C. annuum.





Similar content being viewed by others
References
M.J. Adams, J.F. Antoniw, J. Kreuze, Virgaviridae: a new family of rod-shaped plant viruses. Arch. Virol. 154, 1967–1972 (2009)
M. Asano, R. Satoh, A. Mochizuki, S. Tsuda, T. Yamanaka, M. Nishiguchi, K. Hirai, T. Meshi, S. Naito, M. Ishikawa, Tobamovirus-resistant tobacco generated by RNA interference directed against host genes. FEBS Lett. 579, 4479–4484 (2005)
M. Bendahmane, J. Szecsi, I. Chen, R.H. Berg, R.N. Beachy, Characterization of mutant tobacco mosaic virus coat protein that interferes with virus cell-to-cell movement. Proc. Natl. Acad. Sci. USA 99, 3645–3650 (2002)
J.N. Bragg, A.O. Jackson, The C-terminal region of the Barley stripe mosaic virusgammab protein participates in homologous interactions and is required for suppression of RNA silencing. Mol. Plant Pathol. 5, 465–481 (2004)
J.-D. Cho, J.-S. Kim, S.-H. Lee, G.-S. Choi, B.-N. Chung, Viruses and symptoms on peppers, and their infection types in Korea. Res. Plant Dis. 13, 75–81 (2007)
T. Csorba, A. Bovi, T. Dalmay, J. Burgyan, The p122 subunit of Tobacco Mosaic Virus replicase is a potent silencing suppressor and compromises both small interfering RNA- and microRNA-mediated pathways. J. Virol. 81, 11768–11780 (2007)
M. Deng, J.N. Bragg, S. Ruzin, D. Schichnes, D. King, M.M. Goodin, A.O. Jackson, Role of the sonchus yellow net virus N protein in formation of nuclear viroplasms. J. Virol. 81, 5362–5374 (2007)
P.M. Derrick, S.A. Carter, R.S. Nelson, Mutation of the tobacco mosaic tobamovirus 126-and 183-kDa proteins: effects on phloem-dependent virus accumulation and synthesis of viral proteins. Mol. Plant Microbe Interact. 10, 589–596 (1997)
X.S. Ding, J. Liu, N.H. Cheng, A. Folimonov, Y.M. Hou, Y. Bao, C. Katagi, S.A. Carter, R.S. Nelson, The Tobacco mosaic virus 126-kDa protein associated with virus replication and movement suppresses RNA silencing. Mol. Plant Microbe Interact. 17, 583–592 (2004)
Figueira A. dos Reis, S. Golem, S.P. Goregaoker, J.N. Culver, A nuclear localization signal and a membrane association domain contribute to the cellular localization of the Tobacco mosaic virus 126-kDa replicase protein. Virology 301, 81–89 (2002)
M.M. Goodin, R.G. Dietzgen, D. Schichnes, S. Ruzin, A.O. Jackson, pGD vectors: versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J. 31, 375–383 (2002)
S.P. Goregaoker, D.J. Lewandowski, J.N. Culver, Identification and functional analysis of an interaction between domains of the 126/183-kDa replicase-associated proteins of tobacco mosaic virus. Virology 282, 320–328 (2001)
S.P. Goregaoker, J.N. Culver, Oligomerization and activity of the helicase domain of the tobacco mosaic virus 126- and 183-kilodalton replicase proteins. J. Virol. 77, 3549–3556 (2003)
Y. Hagiwara, K. Komoda, T. Yamanaka, A. Tamai, T. Meshi, R. Funada, T. Tsuchiya, S. Naito, M. Ishikawa, Subcellular localization of host and viral proteins associated with tobamovirus RNA replication. EMBO J. 22, 344–353 (2003)
Y. Hagiwara-Komoda, K. Hirai, A. Mochizuki, M. Nishiguchi, T. Meshi, M. Ishikawa, Overexpression of a host factor TOM1 inhibits tomato mosaic virus propagation and suppression of RNA silencing. Virology 376, 132–139 (2008)
P.A. Harries, K. Palanichelvam, S. Bhat, R.S. Nelson, Tobacco mosaic virus 126-kDa protein increases the susceptibility of Nicotiana tabacum to other viruses and its dosage affects virus-induced gene silencing. Mol. Plant Microbe Interact. 21, 1539–1548 (2008)
M. Heinlein, H.S. Padgett, J.S. Gens, B.G. Pickard, S.J. Casper, B.L. Epel, R.N. Beachy, Changing patterns of localization of the tobacco mosaic virus movement protein and replicase to the endoplasmic reticulum and microtubules during infection. Plant Cell 10, 1107–1120 (1998)
K. Ishibashi, M. Nishikiori, M. Ishikawa, Interactions between tobamovirus replication proteins and cellular factors: their impacts on virus multiplication. Mol. Plant Microbe Interact. 23, 1413–1419 (2010)
M. Ishikawa, T. Meshi, F. Motoyoshi, N. Takamatsu, Y. Okada, In vitro mutagenesis of the putative replicase genes of tobacco mosaic virus. Nucleic Acids Res. 14, 8291–8305 (1986)
J.-S. Kim, J.-H. Kim, G.-S. Choi, S.-Y. Chae, H.-R. Kim, B.-N. Joung, Y.-M. Choi, Characterization of Tobacco mosaic virus isolated from Solanum tuberosum ‘Chubak’ in Korea. Res. Plant Dis. 9, 89–93 (2003)
J.-S. Kim, S.-H. Lee, H.-S. Choi, M.-K. Kim, H.-R. Kwak, J.-S. Kim, M. Nam, J.-D. Cho, I.-S. Cho, G.-S. Choi, 2007–2011 characteristics of plant virus infections on crop samples submitted from agricultural places. Res. Plant Dis. 18, 277–289 (2012)
S.-J. Ko, Y.-H. Lee, K.-H. Cha, J.-W. Park, H.-G. Choi, Detection of CGMMV from commercial cucumber seed and resistance test of cultivars. Res. Plant Dis. 10(2), 154–158 (2004)
K. Kubota, S. Tsuda, A. Tamai, T. Meshi, Tomato mosaic virus replication protein suppresses virus-targeted posttranscriptional gene silencing. J. Virol. 77, 11016–11026 (2003)
S. Kumar, A.C. Udaya Shankar, S.C. Nayaka, O.S. Lund, H.S. Prakash, Detection of tobacco mosaic virus and Tomato mosaic virus in pepper and tomato by multiplex RT-PCR. Lett. Appl. Microbiol. 53, 359–363 (2011)
Y. Kurihara, N. Inaba, N. Kutsuna, A. Takeda, Y. Tagami, Y. Watanabe, Binding of tobamovirus replication protein with small RNA duplexes. J. Gen. Virol. 88, 2347–2352 (2007)
S.-H. Lee, J.-B. Lee, S.-M. Kim, H.-S. Choi, J.-W. Park, J.-S. Lee, K.-W. Lee, J.-S. Moon, The incidence and distribution of viral diseases in pepper by cultivation types. Res. Plant Dis. 10, 231–240 (2004)
H.S. Lim, T.S. Ko, K.N. Lambert, H.G. Kim, S.S. Korban, G.L. Hartman, L.L. Domier, Soybean mosaic virus helper component-protease enhances somatic embryo production and stabilizes transgene expression in soybean. Plant Physiol. Biochem. 43, 1014–1021 (2005)
J.Z. Liu, E.B. Blancaflor, R.S. Nelson, The tobacco mosaic virus 126-kilodalton protein, a constituent of the virus replication complex, alone or within the complex aligns with and traffics along microfilaments. Plant Physiol. 138, 1853–1865 (2005)
H. Liu, L. Luo, J. Li, P. Liu, X. Chen, J. Hao, Pollen and seed transmission of Cucumber green mottle mosaic virus in cucumber. Plant. Pathol. 63, 72–77 (2014)
M. Nishikiori, K. Dohi, M. Mori, T. Meshi, S. Naito, M. Ishikawa, Membrane-bound tomato mosaic virus replication proteins participate in RNA synthesis and are associated with host proteins in a pattern distinct from those that are not membrane bound. J. Virol. 80, 8459–8468 (2006)
A. Otuka, Migration of rice planthoppers and their vectored re-emerging and novel rice viruses in East Asia. Viruses Threat. Stable Prod. Cereal Crops 4, 54 (2015)
C.-H. Park, H.-K. Ju, J.-Y. Han, J.-S. Park, I.-H. Kim, E.-Y. Seo, J.-K. Kim, J. Hammond, H.-S. Lim, Complete nucleotide sequences and construction of full-length infectious cDNA clones of Cucumber green mottle mosaic virus (CGMMV) in a versatile newly developed binary vector including both 35S and T7 promoters. Virus Genes (2016)
N. Takamatsu, M. Ishikawa, T. Meshi, Y. Okada, Expression of bacterial chloramphenicol acetyltransferase gene in tobacco plants mediated by TMV-RNA. EMBO J. 6, 307–311 (1987)
K. Tamura, G. Stecher, D. Peterson, A. Filipski, S. Kumar, MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013)
F. Tena, M. Molina-Galdeano, M.T. Serra, I. Garcia-Luque, A single amino acid in the helicase domain of PMMoV-S is responsible for its enhanced accumulation in C. chinense (L3L3) plants at 32 °C. Virology 427, 34–43 (2012)
S. Tsuda, K. Kubota, A. Kanda, T. Ohki, T. Meshi, Pathogenicity of Pepper mild mottle virus is controlled by the rna silencing suppression activity of its replication protein but not the viral accumulation. Phytopathology 97, 412–420 (2007)
T. Watanabe, A. Honda, A. Iwata, S. Ueda, T. Hibi, A. Ishihama, Isolation from tobacco mosaic virus-infected tobacco of a solubilized template-specific RNA-dependent RNA polymerase containing a 126/183 K protein heterodimer. J. Virol. 73, 2633–2640 (1999)
T. Yamanaka, T. Ohta, M. Takahashi, T. Meshi, R. Schmidt, C. Dean, S. Naito, M. Ishikawa, TOM1, an Arabidopsis gene required for efficient multiplication of a tobamovirus, encodes a putative transmembrane protein. Proc. Natl. Acad. Sci. 97, 10107–10112 (2000)
T. Yamanaka, T. Imai, R. Satoh, A. Kawashima, M. Takahashi, K. Tomita, K. Kubota, T. Meshi, S. Naito, M. Ishikawa, Complete inhibition of tobamovirus multiplication by simultaneous mutations in two homologous host genes. J. Virol. 76, 2491–2497 (2002)
Acknowledgements
This research was supported by Technology Commercialization Support Program (Project No. 113044–3), Ministry of Agriculture, Food and Rural Affairs.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Edited by Thomas Hohn.
Sang-Hyuk Han, Jong-Seo Park and Jae-Yeong Han have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Han, SH., Park, JS., Han, JY. et al. New Korean isolates of Pepper mild mottle virus (PMMoV) differ in symptom severity and subcellular localization of the 126 kDa protein. Virus Genes 53, 434–445 (2017). https://doi.org/10.1007/s11262-017-1432-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-017-1432-4