Abstract
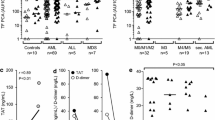
Haemostatic complication is common for patients with hematologic malignancies. Recent studies suggest that the procoagulant activity (PCA) of extracellular vesicles (EV) may play a major role in venous thromboembolism and disseminated intravascular coagulation (DIC) in acute leukaemia. To study the impact of EVs from leukaemic patients on thrombin generation and to assess EV-PCA as a potential biomarker for thrombotic complications in patients with acute leukaemia. Blood samples from a cohort of patients with newly diagnosed acute leukaemia were obtained before treatment (D-0), 3 and 7 days after treatment (D-3 and D-7). Extracellular vesicles were isolated and concentrated by ultracentrifugation. EV-PCA was assessed by thrombin generation assay, and EV-associated tissue factor activity was measured using a commercial bio-immunoassay (Zymuphen MP-TF®). Of the 53 patients, 6 had increased EV-PCA at D-0 and 4 had a thrombotic event. Patients without thrombotic events (n = 47) had no elevated EV-PCA. One patient had increased EVs with procoagulant activity at D-3 and developed a DIC at D-5. This patient had no increased EVs-related tissue factor activity from D-0 to D-7 (<2 pg/ml). Eight patients had increased EVs with tissue factor activity (>2 pg/ml), of these, four had a thrombosis and two had haemorrhages. Procoagulant activity of extracellular vesicles could have a predictive value in excluding the risk of thrombotic events. Our findings also suggest a possible association between thrombotic events and EV-PCA.




Similar content being viewed by others
Abbreviations
- VTE:
-
Venous thromboembolism
- DIC:
-
Disseminated intravascular coagulation
- AML:
-
Acute myeloblastic leukaemia
- ALL:
-
Acute lymphoblastic leukaemia
- EVs:
-
Extracellular vesicles
- TF:
-
Tissue factor
- PL:
-
Procoagulant phospholipids
- PCA:
-
Procoagulant activity
- TGA:
-
Thrombin generation assay
- BMI:
-
Body mass index
- D-0:
-
Before treatment
- D-3:
-
3 days after treatment
- D-7:
-
7 days after treatment
- NPP:
-
Normal pooled plasma
- UC:
-
Ultracentrifugation
- SD:
-
Standard deviation
- CTL:
-
Healthy volunteers control
- TFPI:
-
Tissue factor pathway inhibitor
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
References
Ku GH, White RH, Chew HK, Harvey DJ, Zhou H, Wun T (2009) Venous thromboembolism in patients with acute leukemia: incidence, risk factors, and effect on survival. Blood 113: 3911–3917. doi: 10.1182/blood-2008-08-175745
Kwaan HC, Rego EM (2010) Role of microparticles in the hemostatic dysfunction in acute promyelocytic leukemia. Semin Thromb Hemost 36:917–924
Khorana AA (2013) Venous thromboembolism prevention in cancer outpatients. J Natl Compr Cancer Netw 11:1431–1438
Khorana AA, Dalal M, Lin J, Connolly GC (2013) Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 119:648–655
Davila M, Amirkhosravi A, Coll E, Desai H, Robles L, Colon J, Baker CH, Francis JL. Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. J Thromb Haemost 6:1517–1524. doi: 10.1111/j.1538-7836.2008.02987.x
Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S 2007 Microparticle-associated tissue factor activity: a link between cancer and thrombosis?. J Thromb Haemost 5:520–527. doi: 10.1111/j.1538-7836.2007.02369.x
Pollyea DA, Kohrt HE, Medeiros BC. Acute myeloid leukaemia in the elderly: a review. Br J Haematol 152:524–542
Schneider P, Van Dreden P, Rousseau A, Kassim Y, Legrand E, Vannier JP, Vasse M. Increased levels of tissue factor activity and procoagulant phospholipids during treatment of children with acute lymphoblastic leukaemia. Br J Haematol 148:582–592
Gheldof D, Mullier F, Bailly N, Devalet B, Dogne JM, Chatelain B, Chatelain C (2014) Microparticle bearing tissue factor: a link between promyelocytic cells and hypercoagulable state. Thromb Res 133:433–439. doi: 10.1016/j.thromres.2013.11.008
Furie B, Furie BC (2008) Mechanisms of thrombus formation. New Engl J Med 359:938–949. doi: 10.1056/NEJMra0801082
Mackman N (2004) Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol 24:1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74
Zhou J, Shi J, Hou J, Cao F, Zhang Y, Rasmussen JT, Heegaard CW, Gilbert GE (2010) Phosphatidylserine exposure and procoagulant activity in acute promyelocytic leukemia. J Thromb Haemost 8:773–782. doi: 10.1111/j.1538-7836.2010.03763.x
Thaler J, Pabinger I, Sperr WR, Ay C (2014) Clinical evidence for a link between microparticle-associated tissue factor activity and overt disseminated intravascular coagulation in patients with acute myelocytic leukemia. Thromb Res 133:303–305. doi: 10.1016/j.thromres.2013.12.029
Issman L, Brenner B, Talmon Y, Aharon A (2013) Cryogenic transmission electron microscopy nanostructural study of shed microparticles. PloS ONE 8:e83680
Waisman D, Danino D, Weintraub Z, Schmidt J, Talmon Y (2007) Nanostructure of the aqueous form of lung surfactant of different species visualized by cryo-transmission electron microscopy. Clin Physiol Funct Imaging 27:375–380
Tatischeff I, Larquet E, Falcon-Perez JM, Turpin PY, Kruglik SG (2012) Fast characterisation of cell-derived extracellular vesicles by nanoparticles tracking analysis, cryo-electron microscopy, and Raman tweezers microspectroscopy. J Extracell Vesicles. doi: 10.3402/jev.v1i0.19179
Gheldof D, Hardij J, Cecchet F, Chatelain B, Dogne JM, Mullier F (2013) Thrombin generation assay and transmission electron microscopy: a useful combination to study tissue factor-bearing microvesicles. J Extracell Vesicles. doi: 10.3402/jev.v2i0.19728
Gheldof D, Mullier F, Chatelain B, Dogne JM, Chatelain C (2013) Inhibition of tissue factor pathway inhibitor increases the sensitivity of thrombin generation assay to procoagulant microvesicles. Blood Coagul Fibrinolysis 24:567–572. doi: 10.1097/MBC.0b013e328360a56e
Walter RB, Othus M, Burnett AK, Lowenberg B, Kantarjian HM, Ossenkoppele GJ, Hills RK, van Montfort KG, Ravandi F, Evans A, Pierce SR, Appelbaum FR, Estey EH (2013) Significance of FAB subclassification of “acute myeloid leukemia, NOS” in the 2008 WHO classification: analysis of 5848 newly diagnosed patients. Blood 121:2424–2431. doi: 10.1182/blood-2012-10-462440
Carrier M, Khorana AA, Zwicker JI, Noble S, Lee AY (2014) Subcommittee on H, Malignancy for the SSCotI. Management of challenging cases of patients with cancer-associated thrombosis including recurrent thrombosis and bleeding: guidance from the SSC of the ISTH: a reply to a rebuttal. J Thromb Haemost 12:116–117. doi: 10.1111/jth.12444
Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, Subcommittee on Control of A (2015) Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost 13:2119–2126
Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M, Scientific Subcommittee on Disseminated Intravascular Coagulation of the International Society on T, Haemostasis (2001) Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost 86:1327–1330
Knobl PN (2005) On the evaluation of the ISTH score for overt disseminated intravascular coagulation. Crit Care Med 33:1185–1186
Schultz KR, Massing B, Spinelli JJ, Gaynon PS, Wadsworth L (1997) Importance of the day 7 bone marrow biopsy as a prognostic measure of the outcome in children with acute lymphoblastic leukemia. Med Pediatr Oncol 29:16–22
Robert S, Ghiotto J, Pirotte B, David JL, Masereel B, Pochet L, Dogne JM (2009) Is thrombin generation the new rapid, reliable and relevant pharmacological tool for the development of anticoagulant drugs?. Pharmacol Res 59:160–166. doi: 10.1016/j.phrs.2008.12.003
Mullier F, Bailly N, Chatelain C, Chatelain B, Dogne JM (2013) Pre-analytical issues in the measurement of circulating microparticles: current recommendations and pending questions. J Thromb Haemost 11(4):693–696
Hemker HC, Giesen P, AlDieri R, Regnault V, de Smed E, Wagenvoord R, Lecompte T, Beguin S (2002) The calibrated automated thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb 32: 249–253
Khorana AA, Connolly GC (2009) Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol 27:4839–4847. doi: 10.1200/JCO.2009.22.3271
Owens AP 3rd, Mackman N (2012) Microparticles in hemostasis and thrombosis. Circ Res 108:1284–1297
Lima LG, Oliveira AS, Campos LC, Bonamino M, Chammas R, Werneck C, Vicente CP, Barcinski MA, Petersen LC, Monteiro RQ (2011) Malignant transformation in melanocytes is associated with increased production of procoagulant microvesicles. Thromb Haemost 106:712–723
Li M, Yu D, Williams KJ, Liu ML (2010) Tobacco smoke induces the generation of procoagulant microvesicles from human monocytes/macrophages. Arterioscler Thromb Vasc Biol 30:1818–1824
Van Aalderen MC, Trappenburg MC, Van Schilfgaarde M, Molenaar PJ, Ten Cate H, Terpstra WE, Leyte A (2011) Procoagulant myeloblast-derived microparticles in AML patients: changes in numbers and thrombin generation potential during chemotherapy. J Thromb Haemost 9:223–226. doi: 10.1111/j.1538-7836.2010.04133.x
Langer F, Amirkhosravi A, Loges S, Meyer T, Eifrig B, Hossfeld DK, Fiedler W, Francis JL (2004) An in vitro study on the mechanisms of coagulation activation in acute myelogenous leukemia (AML): role of tissue factor regulation by cytotoxic drugs and GM-CSF. Thromb Haemost 92:1136–1146
Langer F, Ruf W (2014) Synergies of phosphatidylserine and protein disulfide isomerase in tissue factor activation. Thromb Haemost. 111:590–597
Tormoen GW, Recht O, Gruber A, Levine RL, McCarty OJ (2013) Phosphatidylserine index as a marker of the procoagulant phenotype of acute myelogenous leukemia cells. Phys Biol 10:056010
Marchetti M, Russo L, Balducci D, Falanga A (2011) All trans-retinoic acid modulates the procoagulant activity of human breast cancer cells. Thromb Res 128:368–374
Zhang XH, Hu Y, Hong M, Xia LH, Guo T, Shen GX, Wei WN, Song SJ (2007) Effects of arsenic trioxide or retinoic acid on mRNA and protein expression of tissue factor and thrombomodulin and procoagulant activity in NB4 cells. J Exp Hematol Chin Assoc Pathophysiol 15:391–395
Hellum M, Ovstebo R, Troseid AM, Berg JP, Brandtzaeg P, Henriksson CE (2012) Microparticle-associated tissue factor activity measured with the Zymuphen MP-TF kit and the calibrated automated thrombogram assay. Blood Coagul 23: 520–526. doi: 10.1097/MBC.0b013e328354a256
Tatsumi K, Antoniak S, Monroe DM, Khorana AA, Mackman N, Subcommittee on H, Malignancy of the S, Standardization Committee of the International Society on T, Hemostasis (2014) Evaluation of a new commercial assay to measure microparticle tissue factor activity in plasma: communication from the SSC of the ISTH. J Thromb Haemost 12:1932–1934. doi: 10.1111/jth.12718
Gheldof D, Chatelain C, Dogne JM, Mullier F (2014) Microparticle-associated tissue factor activity and overt disseminated intravascular coagulation in patients with acute myelocytic leukemia. Thromb Res 134: 213–214. doi: 10.1016/j.thromres.2014.05.001
Acknowledgements
The work was supported by Grant No 7.4545.11 from F.R.S. - FNRS Télévie Belgium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interests.
Rights and permissions
About this article
Cite this article
Gheldof, D., Haguet, H., Dogné, JM. et al. Procoagulant activity of extracellular vesicles as a potential biomarker for risk of thrombosis and DIC in patients with acute leukaemia. J Thromb Thrombolysis 43, 224–232 (2017). https://doi.org/10.1007/s11239-016-1471-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-016-1471-z