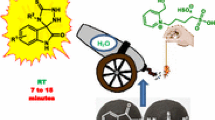
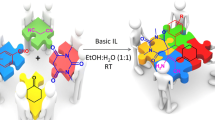
Abstract
We have designed and synthesized an efficient novel task-specific ionic liquid, 1-(ethylaceto acetate)-1-(2-hydroxyethyl) piperidinium tetrachloroaluminate [EAHEPiPY]+ [AlCl4]–. Its catalytic efficiency was explored for multicomponent reaction of isatin(s), malononitrile, and indole(s) furnished corresponding 3,3′-disubstituted oxindoles via tandem Knoevenagel/Michael addition reaction in excellent yield at 80 °C in mixed solvent system, water: ethanol (80:20%).
Graphical Abstract







Similar content being viewed by others
References
R.V.A. Orru, M. de Greef, Synthesis 10, 1471 (2003)
P. Slobbe, E. Ruijter, R.V.A. Orru, Med. Chem. Commun. 3, 1189 (2012)
A. Strecker, Liebigs. Ann. Chem. 75, 27–45 (1850)
I. Ugi, Adv. Synth. Catal. 339, 499 (1997)
N.K. Terret, M. Gardener, D.W. Gordon, R.J. Kobylecki, J. Steele, Tetrahedron 51, 8135 (1995)
A. Domling, I. Ugi, Angew. Chem. Int. Ed. 39, 3168 (2000)
A. Domling, Chem. Rev. 106, 17 (2006)
R.W. Armstrong, A.P. Combs, P.A. Tempest, S.D. Brown, T.A. Keating, Acc. Chem. Res. 29, 123 (1996)
F.L. Muller, T. Constantieux, J. Rodriguez, J. Am. Chem. Soc. 127, 17176 (2005)
B. Willy, T.J.J. Muller, Eur. J. Org. Chem. 2008, 4157 (2008)
M. Adib, E. Sheikhi, A. Kavoosi, H.R. Bijanzadeh, Tetrahedron 66, 9263 (2010)
H.X. Mei, Z. Yang, G. Yun-Fei, X. Yi-Bo, Z. Bi-Xian, Adv. Mat. Res. 554-556, 557 (2012)
J.R. Harjani, S.J. Nara, M.M. Salunkhe, Tetrahedron Lett. 43, 1127 (2002)
Y.-F. Han, M. Xia, Curr. Org. Chem. 14, 379 (2010)
H. Deppermann, A.H. Thomanek, G.P. Prenzel, W.J. Maison, Org. Chem. 75, 5994 (2010)
Z.-Y. Cao, Y.-H. Wang, X.-P. Zeng, J. Zhou, Tetrahedron Lett. 55, 2571 (2014)
S Wang, S Yu, W Sun, S K Shangary, D Sun, P Zou, D McEachern, Y Zhao, U. S. Patent 2,011,112,052 (2011)
G. Zeni, R.C. Larock, Chem. Rev. 106, 4644 (2006)
J.P. Michael, Nat. Prod. Rep. 22, 627 (2005)
F. Zhou, Y.-L. Liu, J Zhou Adv. Synth. Catal. 352, 1381 (2010)
P.G. Mahajan, D.P. Bhopate, G.B. Kolekar, S.R. Patil, Sensor. Actuators B 220, 864 (2015)
M. Kumar, K. Ramasamy, V. Mani, R.K. Mishra, A.B.A. Majeed, E.D. Clercq, B. Narasimhan, Arab. J. Chem. 7, 396 (2014)
D. Havrylyuk, B. Zimenkovsky, O. Vasylenko, A. Gzella, R. Lesyk, J. Med. Chem. 55, 8630 (2012)
C. Liang, J. Xia, D. Lei, X. Li, Q. Yao, J. Gao, Eur. J. Med. Chem. 74, 742 (2014)
S.B. Kumar, M. Ravinder, G. Kishore, V.J. Rao, P. Yogeeswari, D. Sriram, Med. Chem. Res. 23, 1934 (2014)
K. Han, Y. Zhou, F. Liu, Q. Guo, P. Wang, Y. Yang, B. Song, W. Liu, Q. Yao, Y. Teng, P. Yu, Bioorg. Med. Chem. Lett. 24, 591 (2014)
K. Kumar, S. Carrere-Kremer, L. Kremer, Y. Gueerardel, C. Biot, V. Kumar, Organometallics 32, 5713 (2013)
P. Mondal, S. Jana, A. Balaji, R. Ramakrishna, L.K. Kanthal, J. Young Pharm. 4, 38 (2012)
R. Raj, P. Singh, P. Singh, J. Gut, P.J. Rosenthal, V. Kumar, Eur. J. Med. Chem. 62, 590 (2013)
B.K. Paul, D. Ray, N. Guchhait, Phys. Chem. Chem. Phys. 15, 1275 (2013)
G. Kiran, T. Maneshwar, Y. Rajeshwar, M. Sarangapani, J. Chem. 2013, 1 (2013)
T. He, Q.Q. Zeng, D.C. Yang, Y.H. He, Z. Guan, RSC Adv. 5, 37843 (2015)
T. Welton, Chem. Rev. 99, 2071 (1999)
J.P. Hallett, T. Welton, Chem. Rev. 111, 3508 (2011)
P. Wassercheid, W. Keim, Chem. Int. Ed. 39, 3772 (2000)
J.H. Davis, P.A. Fox, Chem. Commun. 11, 1209 (2003)
R. Shedon, Chem. Commun. 23, 2399 (2001)
W. Leitner, Nature 423, 930 (2003)
R.A. Brown, P. Pollet, C.A. Mckert, C.L. Litta, P.G. Jessop, J. Am. Chem. Soc. 123, 1254 (2001)
Z.C. Liu, X.H. Meng, R. Zhang, C.M. Xu, H. Dong, Y.F. Hu, AIChE. J. 60, 2244 (2014)
J. Estager, J.D. Holbrey, M. Swadzba-Kwasny, Chem. Soc. Rev. 43, 847 (2014)
A.L. Zhu, T. Jiang, D. Wang, B.X. Han, L. Liu, J. Huang, J.C. Zhang, D.H. Sun, Green Chem. 7, 514 (2005)
B.C. Ranu, R. Jana, J. Org. Chem. 70, 8621 (2005)
T. Akaiyama, A. Suzuki, K. Fuchibe, Synlett 2005, 1024 (2005)
W. Sun, C.G. Xia, H.W. Wang, Tetrahedron. Lett. 44, 2409 (2003)
K.R. Seddon, A. Stark, M. Torres, J. Pure Appl. Chem. 72, 2275 (2000)
N.G. Khaligh, Monatsh Chem. 145, 1643 (2014)
K.P. Boroujeni, M. Jafarinasab, J. Chem. Res. 36, 429 (2012)
K.P. Boroujeni, M. Jafarinasab, Chin. Chem. Lett. 23, 1067 (2012)
J. Estager, J.D. Holbrey, M. Swadzba-Kwasny, Chem. Soc. Rev. 43, 847 (2014)
H. Xing, T. Wang, Z. Zhou, Y. Dai, Ind. Eng. Chem. Res. 2005, 4147 (2005)
I.C. Quarmby, R.A. Mantz, L.M. Goldenberg, R.A. Osteryoung, Anal. Chem. 66, 3558 (1994)
D.S. Gaikwad, K.A. Undale, T.S. Shaikh, D.M. Pore, C. R. Chimie 14, 865 (2011)
D.M. Pore, P.G. Hegade, D.S. Gaikwad, P.B. Patil, J.D. Patil, Lett. Org. Chem. 11, 131 (2014)
K.A. Undale, T.S. Shaikh, D.S. Gaikwad, D.M. Pore, C. R. Chimie 14, 511 (2011)
D.M. Pore, T.S. Shaikh, K.A. Undale, D.S. Gaikwad, C. R. Chimie 13, 1429 (2010)
J. Sanz, J.M. Serratosa, J. Am. Chem. Soc. 106, 4790 (1984)
M. Mantovani, A. Escudero, A.I. Becerro, Clays Clay Miner. 57, 302 (2009)
K.P. Boroujeni, P. Ghasemi, Cat. Comm. 37, 50 (2013)
Y. Wei, C. Keke, X. Zhang, Y. Kuang, X. Tang, H. Xiaoxiang, J. Ind. Eng. Chem. doi:10.1016/j.jiec.2015.04.002 (2015)
N.C. Dige, D.M. Pore, Synth. Commun. 45, 2498 (2015)
N.C. Dige, J.D. Patil, D.M. Pore, Catal. Lett. 147, 301 (2017)
D.M. Pore, P.B. Patil, D.S. Gaikwad, P.G. Hegade, J.D. Patil, K.A. Undale, Tetrahedron Lett. 54, 5876 (2013)
P.G. Hegade, S.D. Chinchkar, D.M. Pore, Monatsh Chem. 145, 1243 (2016)
Acknowledgements
Author DMP is thankful to the DST Fast Track Scheme, New Delhi for financial assistance [No. SB/FT/CS-154/2012]. One of the authors NCD is grateful to the University Grants Commission (UGC), New Delhi for providing financial assistance in the form of UGC-BSR-SAP fellowship (F.25-1/2014-15(BSR)/7-183/2009(BSR)–5 November 2015).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dige, N.C., Korade, S.N. & Pore, D.M. Design of task-specific ionic liquid, 1-(ethylaceto acetate)-1-(2-hydroxyethyl) piperidinium tetrachloroaluminate for multicomponent synthesis of 3,3′-disubstituted oxindoles. Res Chem Intermed 43, 7029–7040 (2017). https://doi.org/10.1007/s11164-017-3034-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3034-0