Abstract
The increased prevalence of obesity has mandated extensive research focused on mechanisms responsible for associated clinical complications. Emerging from the focus on adipose tissue biology as a vitally important adipokine is adiponectin which is now believed to mediate anti-diabetic, anti-atherosclerotic, anti-inflammatory, cardioprotective and cancer modifying actions. Adiponectin mediates these primarily beneficial effects via direct signaling effects and via enhancing insulin sensitivity via crosstalk with insulin signaling pathways. Reduced adiponectin action is detrimental and occurs in obesity via decreased circulating levels of adiponectin action or development of adiponectin resistance. This review will focus on cellular mechanisms of adiponectin action, their crosstalk with insulin signaling and the resultant role of adiponectin in cardiovascular disease, diabetes and cancer and reviews data from in vitro cell based studies through animal models to clinical observations.


Similar content being viewed by others
References
Vucenik I, Stains JP. Obesity and cancer risk: Evidence, mechanisms, and recommendations. [Review]. Ann N Y Acad Sci. 2012;1271:37–43. doi:10.1111/j.1749-6632.2012.06750.x.
Despres JP. Body fat distribution and risk of cardiovascular disease: An update. [Research support, Non-U.S. Gov’t Review]. Circulation. 2012;126(10):1301–13. doi:10.1161/CIRCULATIONAHA.111.067264.
Harwood Jr HJ. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology. 2012;63(1):57–75. doi:10.1016/j.neuropharm.2011.12.010.
Dadson K, Liu Y, Sweeney G. Adiponectin action: A combination of endocrine and autocrine/paracrine effects. Front Endocrinol (Lausanne). 2011;2:62. doi:10.3389/fendo.2011.00062.
Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: An update. Br J Pharmacol. 2012;165(3):574–90. doi:10.1111/j.1476-5381.2011.01395.x.
Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: A review of current evidence. Endocr Rev. 2012;33(4):547–94. doi:10.1210/er.2011-1015.
Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate Antidiabetic metabolic effects. [Research support, Non-U.S. Gov’t]. Nature. 2003;423(6941):762–9. doi:10.1038/nature01705.
Hall J, Roberts R, Vora N. Energy homoeostasis: The roles of adipose tissue-derived hormones, peptide YY and Ghrelin. Obesity Facts. 2009;2(2):117–25.
Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–51. doi:10.1210/er.2005-0005.
Jäger S, Handschin C, St.-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci. 2007;104(29):12017–22. doi:10.1073/pnas.0705070104.
Nemoto S, Fergusson MM, Finkel T. SIRT1 Functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1Œ±. J Biol Chem. 2005;280(16):16456–60. doi:10.1074/jbc.M501485200.
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–8. doi:10.1038/nature03354.
Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD + metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–60. doi:10.1038/nature07813.
Hardie DG, Alessi DR. LKB1 And AMPK and the cancer-metabolism link—ten years after. BMC Biol. 2013;11:36. doi:10.1186/1741-7007-11-36.
Zeqiraj E, Filippi BM, Deak M, Alessi DR, van Aalten DM. Structure of the LKB1-STRAD-MO25 complex reveals an allosteric mechanism of kinase activation. Science. 2009;326(5960):1707–11. doi:10.1126/science.1178377.
Boudeau J, Baas AF, Deak M, Morrice NA, Kieloch A, Schutkowski M, et al. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003;22(19):5102–14. doi:10.1093/emboj/cdg490.
Baas AF, Boudeau J, Sapkota GP, Smit L, Medema R, Morrice NA, et al. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J. 2003;22(12):3062–72. doi:10.1093/emboj/cdg292.
Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2(1):21–33. doi:10.1016/j.cmet.2005.06.005.
Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2(1):9–19. doi:10.1016/j.cmet.2005.05.009.
Herrero-Martin G, Hoyer-Hansen M, Garcia-Garcia C, Fumarola C, Farkas T, Lopez-Rivas A, et al. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 2009;28(6):677–85. doi:10.1038/emboj.2009.8.
Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281(35):25336–43. doi:10.1074/jbc.M604399200.
Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17(1):113–24.
Dandapani M, Hardie DG. AMPK: Opposing the metabolic changes in both tumour cells and inflammatory cells? Biochem Soc Trans. 2013;41(2):687–93. doi:10.1042/bst20120351.
Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. [Research support, Non-U.S. Gov’t]. Nature. 2010;464(7293):1313–9. doi:10.1038/nature08991.
Wang C, Mao X, Wang L, Liu M, Wetzel MD, Guan KL, et al. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. The Journal of biological chemistry. 2007;282(11):7991–6. doi:10.1074/jbc.M700098200.
Moore T, Beltran L, Carbajal S, Strom S, Traag J, Hursting SD, et al. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer prevention research. 2008;1(1):65–76. doi:10.1158/1940-6207.capr-08-0022.
Taliaferro-Smith L, Nagalingam A, Zhong D, Zhou W, Saxena NK, Sharma D. LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene. 2009;28(29):2621–33. doi:10.1038/onc.2009.129.
Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, et al. APPL1 binds to adiponectin receptors and mediates adiponectin signaling and function. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Nature cell biology. 2006;8(5):516–23. doi:10.1038/ncb1404.
Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6(1):55–68. doi:10.1016/j.cmet.2007.06.003.
Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature medicine. 2002;8(11):1288–95. doi:10.1038/nm788.
Zhou L, Deepa SS, Etzler JC, Ryu J, Mao X, Fang Q, et al. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and Phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J Biol Chem. 2009;284(33):22426–35. doi:10.1074/jbc.M109.028357.
Mitsuuchi Y, Johnson SW, Sonoda G, Tanno S, Golemis EA, Testa JR. Identification of a chromosome 3p14.3-21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene. 1999;18(35):4891–8. doi:10.1038/sj.onc.1203080.
Boudeau J, Scott JW, Resta N, Deak M, Kieloch A, Komander D, et al. Analysis of the LKB1-STRAD-MO25 complex. J Cell Sci. 2004;117(Pt 26):6365–75. doi:10.1242/jcs.01571.
Xie Z, Dong Y, Zhang J, Scholz R, Neumann D, Zou MH. Identification of the serine 307 of LKB1 as a novel phosphorylation site essential for its nucleocytoplasmic transport and endothelial cell angiogenesis. Mol Cell Biol. 2009;29(13):3582–96. doi:10.1128/mcb.01417-08.
Deepa SS, Zhou L, Ryu J, Wang C, Mao X, Li C, et al. APPL1 Mediates adiponectin-induced LKB1 cytosolic localization through the PP2A-PKCŒ∂ signaling pathway. Mol Endocrinol. 2011;25(10):1773–85. doi:10.1210/me.2011-0082.
Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129(7):1261–74. doi:10.1016/j.cell.2007.06.009.
Scheid MP, Woodgett JR. Unravelling the activation mechanisms of protein kinase B/Akt. FEBS Lett. 2003;546(1):108–12.
Patel SA, Hoehn KL, Lawrence RT, Sawbridge L, Talbot NA, Tomsig JL, et al. Overexpression of the adiponectin receptor AdipoR1 in rat skeletal muscle amplifies local insulin sensitivity. Endocrinology. 2012;153(11):5231–46. doi:10.1210/en.2012-1368.
Maruyama S, Shibata R, Ohashi K, Ohashi T, Daida H, Walsh K, et al. Adiponectin ameliorates doxorubicin-induced cardiotoxicity through Akt protein-dependent mechanism. [Research Support, Non-U.S. Gov’t]. The Journal of biological chemistry. 2011;286(37):32790–800. doi:10.1074/jbc.M111.245985.
Saito T, Jones CC, Huang S, Czech MP, Pilch PF. The interaction of Akt with APPL1 is required for insulin-stimulated Glut4 translocation. J Biol Chem. 2007;282(44):32280–7. doi:10.1074/jbc.M704150200.
Cheng KKY, Iglesias MA, Lam KSL, Wang Y, Sweeney G, Zhu W, et al. APPL1 Potentiates Insulin-Mediated Inhibition of Hepatic Glucose Production and Alleviates Diabetes via Akt Activation in Mice. Cell Metab. 2009;9(5):417–27.
Wijesekara N, Krishnamurthy M, Bhattacharjee A, Suhail A, Sweeney G, Wheeler MB. Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J Biol Chem. 2010;285(44):33623–31. doi:10.1074/jbc.M109.085084.
Tsuchida A, Yamauchi T, Ito Y, Hada Y, Maki T, Takekawa S, et al. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem. 2004;279(29):30817–22. doi:10.1074/jbc.M402367200.
Backer JM, Myers Jr MG, Shoelson SE, Chin DJ, Sun XJ, Miralpeix M, et al. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992;11(9):3469–79.
Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev. 2013;23(1):53–62. doi:10.1016/j.gde.2012.12.005.
Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signaling networks. Biochem J. 2012;441(1):1–21. doi:10.1042/bj20110892.
Werner ED, Lee J, Hansen L, Yuan M, Shoelson SE. Insulin resistance Due to phosphorylation of insulin receptor substrate-1 at serine 302. J Biol Chem. 2004;279(34):35298–305. doi:10.1074/jbc.M405203200.
Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, et al. The TSC1-2 tumor suppressor controls insulin‚ÄìPI3K signaling via regulation of IRS proteins. The Journal of Cell Biology. 2004;166(2):213–23. doi:10.1083/jcb.200403069.
Vu V, Kim W, Fang X, Liu YT, Xu A, Sweeney G. Coculture with primary visceral rat adipocytes from control but not Streptozotocin-induced diabetic animals increases glucose uptake in rat skeletal muscle cells: Role of adiponectin. [Research support, Non-U.S. Gov’t]. Endocrinology. 2007;148(9):4411–9. doi:10.1210/en.2007-0020.
Gaddam KK, Ventura HO, Lavie CJ. Metabolic syndrome and heart failure–the risk, paradox, and treatment. [Review]. Curr Hypertens Rep. 2011;13(2):142–8. doi:10.1007/s11906-011-0179-x.
Anker SD, von Haehling S. The obesity paradox in heart failure: Accepting reality and making rational decisions. [Research support, Non-U.S. Gov’t Review]. Clin Pharmacol Ther. 2011;90(1):188–90. doi:10.1038/clpt.2011.72.
Park M, Sweeney G. Direct effects of adipokines on the heart: Focus on adiponectin. Heart Fail Rev. 2013. doi:10.1007/s10741-012-9337-8.
Fujita K, Maeda N, Sonoda M, Ohashi K, Hibuse T, Nishizawa H, et al. Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler Thromb Vasc Biol. 2008;28(5):863–70.
Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11(10):1096–103.
Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10(12):1384–9.
Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci WS, et al. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007;42(6):1065–74.
Sam F, Duhaney TA, Sato K, Wilson RM, Ohashi K, Sono-Romanelli S, et al. Adiponectin deficiency, diastolic dysfunction, and diastolic heart failure. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Endocrinology. 2010;151(1):322–31. doi:10.1210/en.2009-0806.
Shimano M, Ouchi N, Shibata R, Ohashi K, Pimentel DR, Murohara T, et al. Adiponectin deficiency exacerbates cardiac dysfunction following pressure overload through disruption of an AMPK-dependent angiogenic response. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. J Mol Cell Cardiol. 2010;49(2):210–20. doi:10.1016/j.yjmcc.2010.02.021.
Leroith D. Pathophysiology of the metabolic syndrome: implications for the cardiometabolic risks associated with type 2 diabetes. [Review]. Am J Med Sci. 2012;343(1):13–6. doi:10.1097/MAJ.0b013e31823ea214.
Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88(2):389–419.
Despres JP, Cartier A, Cote M, Arsenault BJ. The concept of cardiometabolic risk: Bridging the fields of diabetology and cardiology. [Research Support, Non-U.S. Gov’t Review]. Ann Med. 2008;40(7):514–23. doi:10.1080/07853890802004959.
Fang X, Palanivel R, Cresser J, Schram K, Ganguly R, Thong FS, et al. An APPL1-AMPK signaling axis mediates beneficial metabolic effects of adiponectin in the heart. [In Vitro Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Am J Physiol Endocrinol Metab. 2010;299(5):E721–729. doi:10.1152/ajpendo.00086.2010.
Palanivel R, Fang X, Park M, Eguchi M, Pallan S, De Girolamo S, et al. Globular and full-length forms of adiponectin mediate specific changes in glucose and fatty acid uptake and metabolism in cardiomyocytes. Cardiovasc Res. 2007;75(1):148–57.
Park M, Youn B, Zheng XL, Wu D, Xu A, Sweeney G. Globular adiponectin, acting via AdipoR1/APPL1, protects H9c2 cells from hypoxia/reoxygenation-induced apoptosis. [Research Support, Non-U.S. Gov’t]. PLoS One. 2011;6(4):e19143. doi:10.1371/journal.pone.0019143.
Wang C, Xin X, Xiang R, Ramos FJ, Liu M, Lee HJ, et al. Yin-Yang regulation of adiponectin signaling by APPL isoforms in muscle cells. J Biol Chem. 2009;284(46):31608–15. doi:10.1074/jbc.M109.010355.
Ebert T, Fasshauer M. Adiponectin: Sometimes good, sometimes bad? Cardiology. 2011;118(4):236–7. doi:10.1159/000329647.
Khan RS, Kato TS, Chokshi A, Chew M, Yu S, Wu C, et al. Adipose tissue inflammation and adiponectin resistance in patients with advanced heart failure: Correction after ventricular assist device implantation. Circ Heart Fail. 2012;5(3):340–8. doi:10.1161/CIRCHEARTFAILURE.111.964031.
Skurk C, Wittchen F, Suckau L, Witt H, Noutsias M, Fechner H, et al. Description of a local cardiac adiponectin system and its deregulation in dilated cardiomyopathy. [Research Support, Non-U.S. Gov’t]. Eur Heart J. 2008;29(9):1168–80. doi:10.1093/eurheartj/ehn136.
Ma Y, Liu Y, Liu S, Qu Y, Wang R, Xia C, et al. Dynamic alteration of adiponectin/adiponectin receptor expression and its impact on myocardial ischemia/reperfusion in type 1 diabetic mice. Am J Physiol Endocrinol Metab. 2011;301(3):E447–455. doi:10.1152/ajpendo.00687.2010.
Wang T, Qiao S, Lei S, Liu Y, Ng KF, Xu A, et al. N-acetylcysteine and allopurinol synergistically enhance cardiac adiponectin content and reduce myocardial reperfusion injury in diabetic rats. PLoS One. 2011;6(8):e23967. doi:10.1371/journal.pone.0023967.
Cui XB, Wang C, Li L, Fan D, Zhou Y, Wu D, et al. Insulin decreases myocardial adiponectin receptor 1 expression via PI3K/Akt and FoxO1 pathway. [Research Support, Non-U.S. Gov’t]. Cardiovasc Res. 2012;93(1):69–78. doi:10.1093/cvr/cvr273.
Fang X, Palanivel R, Zhou X, Liu Y, Xu A, Wang Y, et al. Hyperglycemia-and hyperinsulinemia-induced alteration of adiponectin receptor expression and adiponectin effects in L6 myoblasts. [Research Support, Non-U.S. Gov’t]. J Mol Endocrinol. 2005;35(3):465–76. doi:10.1677/jme.1.01877.
Yi W, Sun Y, Gao E, Wei X, Lau WB, Zheng Q, et al. Reduced cardioprotective action of adiponectin in high-fat diet-induced type II diabetic mice and its underlying mechanisms. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Antioxid Redox Signal. 2011;15(7):1779–88. doi:10.1089/ars.2010.3722.
Wang C, Li L, Zhang ZG, Fan D, Zhu Y, Wu LL. Globular adiponectin inhibits angiotensin II-induced nuclear factor kappaB activation through AMP-activated protein kinase in cardiac hypertrophy. [Research Support, Non-U.S. Gov’t]. J Cell Physiol. 2010;222(1):149–55. doi:10.1002/jcp.21931.
Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, et al. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56(5):1387–94. doi:10.2337/db06-1580.
Wu X, Mahadev K, Fuchsel L, Ouedraogo R, Xu SQ, Goldstein BJ. Adiponectin suppresses IkappaB kinase activation induced by tumor necrosis factor-alpha or high glucose in endothelial cells: role of cAMP and AMP kinase signaling. Am J Physiol Endocrinol Metab. 2007;293(6):E1836–1844. doi:10.1152/ajpendo.00115.2007.
Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102(11):1296–301.
Wang Y, Cheng KK, Lam KS, Wu D, Huang Y, Vanhoutte PM, et al. APPL1 counteracts obesity-induced vascular insulin resistance and endothelial dysfunction by modulating the endothelial production of nitric oxide and endothelin-1 in mice. Diabetes. 2011;60(11):3044–54. doi:10.2337/db11-0666.
Schmid PM, Resch M, Steege A, Fredersdorf-Hahn S, Stoelcker B, Birner C, et al. Globular and full-length adiponectin induce NO-dependent vasodilation in resistance arteries of Zucker lean but not Zucker diabetic fatty rats. Am J Hypertens. 2011;24(3):270–7. doi:10.1038/ajh.2010.239.
Turer AT, Scherer PE. Adiponectin: Mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–26. doi:10.1007/s00125-012-2598-x.
Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013;17(2):185–96. doi:10.1016/j.cmet.2013.01.001.
Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101(28):10308–13. doi:10.1073/pnas.0403382101.
Ordelheide, A. M., Heni, M., Gommer, N., Gasse, L., Haas, C., Guirguis, A., et al. The myocyte expression of adiponectin receptors and PPARdelta is highly coordinated and reflects lipid metabolism of the human donors. [Research Support, Non-U.S. Gov’t]. Exp Diabetes Res, 2011, 692536, doi:10.1155/2011/692536.
Ceddia RB, Somwar R, Maida A, Fang X, Bikopoulos G, Sweeney G. Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells. Diabetologia. 2005;48(1):132–9. doi:10.1007/s00125-004-1609-y.
Liu Y, Chewchuk S, Lavigne C, Brule S, Pilon G, Houde V, et al. Functional significance of skeletal muscle adiponectin production, changes in animal models of obesity and diabetes, and regulation by rosiglitazone treatment. Am J Physiol Endocrinol Metab. 2009;297(3):E657–664. doi:10.1152/ajpendo.00186.2009.
Wang Y, Zhou M, Lam KS, Xu A. Protective roles of adiponectin in obesity-related fatty liver diseases: Mechanisms and therapeutic implications. Arq Bras Endocrinol Metabol. 2009;53(2):201–12.
Ma K, Cabrero A, Saha PK, Kojima H, Li L, Chang BH, et al. Increased beta -oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem. 2002;277(38):34658–61. doi:10.1074/jbc.C200362200.
Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nature medicine. 2002;8(7):731–7. doi:10.1038/nm724.
Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277(29):25863–6. doi:10.1074/jbc.C200251200.
Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–9. doi:10.1038/nm1557.
Bjursell M, Ahnmark A, Bohlooly YM, William-Olsson L, Rhedin M, Peng XR, et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56(3):583–93. doi:10.2337/db06-1432.
Cleasby ME, Lau Q, Polkinghorne E, Patel SA, Leslie SJ, Turner N, et al. The adaptor protein APPL1 increases glycogen accumulation in rat skeletal muscle through activation of the PI3-kinase signaling pathway. J Endocrinol. 2011. doi:10.1530/JOE-11-0039.
Tan Y, You H, Wu C, Altomare DA, Testa JR. Appl1 is dispensable for mouse development, and loss of Appl1 has growth factor-selective effects on Akt signaling in murine embryonic fibroblasts. J Biol Chem. 2010;285(9):6377–89. doi:10.1074/jbc.M109.068452.
Qiao L, Kinney B, Yoo HS, Lee B, Schaack J, Shao J. Adiponectin increases skeletal muscle mitochondrial biogenesis by suppressing mitogen-activated protein kinase phosphatase-1. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Diabetes. 2012;61(6):1463–70. doi:10.2337/db11-1475.
Civitarese AE, Ukropcova B, Carling S, Hulver M, DeFronzo RA, Mandarino L, et al. Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab. 2006;4(1):75–87. doi:10.1016/j.cmet.2006.05.002.
Vu V, Riddell MC, Sweeney G. Circulating adiponectin and adiponectin receptor expression in skeletal muscle: Effects of exercise. Diabetes Metab Res Rev. 2007;23(8):600–11. doi:10.1002/dmrr.778.
Marinho R, Ropelle ER, Cintra DE, De Souza CT, Da Silva AS, Bertoli FC, et al. Endurance exercise training increases APPL1 expression and improves insulin signaling in the hepatic tissue of diet-induced obese mice, independently of weight loss. [Research Support, Non-U.S. Gov’t]. J Cell Physiol. 2012;227(7):2917–26. doi:10.1002/jcp.23037.
Farias JM, Maggi RM, Tromm CB, Silva LA, Luciano TF, Marques SO, et al. Exercise training performed simultaneously to a high-fat diet reduces the degree of insulin resistance and improves adipoR1-2/APPL1 protein levels in mice. [Research Support, Non-U.S. Gov’t]. Lipids Health Dis. 2012;11:134. doi:10.1186/1476-511X-11-134.
Holmes RM, Yi Z, De Filippis E, Berria R, Shahani S, Sathyanarayana P, et al. Increased abundance of the adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif (APPL1) in patients with obesity and type 2 diabetes: evidence for altered adiponectin signaling. Diabetologia. 2011. doi:10.1007/s00125-011-2173-x.
Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325–37. doi:10.1038/nrc3038.
Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330(6009):1340–4. doi:10.1126/science.1193494.
VanSaun MN. Molecular pathways: Adiponectin and leptin signaling in cancer. Clin Cancer Res. 2013;19(8):1926–32. doi:10.1158/1078-0432.ccr-12-0930.
Bergstrom A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. International journal of cancer. Journal international du cancer. 2001;91(3):421–30.
Kritchevsky D. Diet and cancer: What’s Next? The Journal of nutrition. 2003;133(11):3827S–9S.
Shehzad A, Iqbal W, Shehzad O, Lee YS. Adiponectin: Regulation of its production and its role in human diseases. Hormones (Athens). 2012;11(1):8–20.
Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271(18):10697–703. doi:10.1074/jbc.271.18.10697.
Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, et al. Circulating concentrations of the adipocyte protein adiponectin Are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50(5):1126–33. doi:10.2337/diabetes.50.5.1126.
Korner A, Pazaitou-Panayiotou K, Kelesidis T, Kelesidis I, Williams CJ, Kaprara A, et al. Total and high-molecular-weight adiponectin in breast cancer: In vitro and in vivo studies. Journal of Clinical Endocrinology & Metabolism. 2007;92(3):1041–8. doi:10.1210/jc.2006-1858.
Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, et al. Adiponectin and breast cancer risk. Journal of Clinical Endocrinology & Metabolism. 2004;89(3):1102–7. doi:10.1210/jc.2003-031804.
Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9(15):5699–704.
Chen D-C, Chung Y-F, Yeh Y-T, Chaung H-C, Kuo F-C, Fu O-Y, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer letters. 2006;237(1):109–14.
Duggan C, Irwin ML, Xiao L, Henderson KD, Smith AW, Baumgartner RN, et al. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J Clin Oncol. 2011;29(1):32–9. doi:10.1200/jco.2009.26.4473.
Dieudonne M-N, Bussiere M, Dos Santos E, Leneveu M-C, Giudicelli Y, Pecquery R. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2006;345(1):271–9. doi:10.1016/j.bbrc.2006.04.076.
Grossmann ME, Nkhata KJ, Mizuno NK, Ray A, Cleary MP. Effects of adiponectin on breast cancer cell growth and signaling. Br J Cancer. 2008;98(2):370–9. doi:10.1038/sj.bjc.6604166.
Li G, Cong L, Gasser J, Zhao J, Chen K, Li F. Mechanisms underlying the anti-proliferative actions of adiponectin in human breast cancer cells, MCF7‚ÄìDependency on the cAMP/protein kinase-a pathway. Nutrition and Cancer. 2010;63(1):80–8. doi:10.1080/01635581.2010.516472.
Pfeiler GH, Buechler C, Neumeier M, Schaffler A, Schmitz G, Ortmann O, et al. Adiponectin effects on human breast cancer cells are dependent on 17-beta estradiol. Oncol Rep. 2008;19(3):787–93.
Taliaferro-Smith L, Nagalingam A, Knight BB, Oberlick E, Saxena NK, Sharma D. Integral role of PTP1B in adiponectin-mediated inhibition of oncogenic actions of leptin in breast carcinogenesis. Neoplasia. 2013;15(1):23–38.
Nkhata KJ, Ray A, Schuster TF, Grossmann ME, Cleary MP. Effects of adiponectin and leptin co-treatment on human breast cancer cell growth. Oncol Rep. 2009;21(6):1611–9.
Kim KY, Baek A, Hwang JE, Choi YA, Jeong J, Lee MS, et al. Adiponectin-activated AMPK stimulates dephosphorylation of AKT through protein phosphatase 2A activation. Cancer Res. 2009;69(9):4018–26. doi:10.1158/0008-5472.can-08-2641.
Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, et al. Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 2006;66(23):11462–70. doi:10.1158/0008-5472.can-06-1969.
Liu J, Lam JB, Chow KH, Xu A, Lam KS, Moon RT, et al. Adiponectin stimulates Wnt inhibitory factor-1 expression through epigenetic regulations involving the transcription factor specificity protein 1. Carcinogenesis. 2008;29(11):2195–202. doi:10.1093/carcin/bgn194.
Lam JB, Chow KH, Xu A, Lam KS, Liu J, Wong NS, et al. Adiponectin haploinsufficiency promotes mammary tumor development in MMTV-PyVT mice by modulation of phosphatase and tensin homolog activities. PLoS One. 2009;4(3):e4968. doi:10.1371/journal.pone.0004968.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. doi:10.1126/science.1160809.
Boudeau JRM, Sapkota G, Alessi DR. LKB1, a protein kinase regulating cell proliferation and polarity. FEBS Lett. 2003;546(1):159–65. doi:10.1016/S0014-5793(03)00642-2.
Kyriakis JM. At the crossroads: AMP-activated kinase and the LKB1 tumor suppressor link cell proliferation to metabolic regulation. J Biol. 2003;2(4):26. doi:10.1186/1475-4924-2-26.
Inoki K, Zhu T, Guan K-L. TSC2 Mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–90.
Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K, et al. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signaling pathway. Genes Cells. 2003;8(1):65–79.
Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer cell. 2004;6(1):91–9.
Hardie DG. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8(10):774–85. doi:10.1038/nrm2249.
Takahata C, Miyoshi Y, Irahara N, Taguchi T, Tamaki Y, Noguchi S. Demonstration of adiponectin receptors 1 and 2 mRNA expression in human breast cancer cells. Cancer Lett. 2007;250(2):229–36. doi:10.1016/j.canlet.2006.10.006.
Karaduman M, Bilici A, Ozet A, Sengul A, Musabak U, Alomeroglu M. Tissue levels of adiponectin in breast cancer patients. Med Oncol. 2007;24(4):361–6.
Acknowledgements
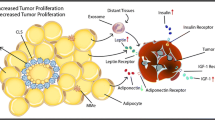
Related work in the authors laboratories has been supported by Canadian Institutes of Health Research, Canadian Diabetes Association and Heart & Stroke Foundation of Canada. GS acknowledges a Career Investigator Award from Heart & Stroke Foundation of Ontario. We thank Hana Kim for graphic design assistance in Fig. 2.
Conflict of interest
The authors declare that there is no conflict of interest pertaining to the content of this review article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scheid, M.P., Sweeney, G. The role of adiponectin signaling in metabolic syndrome and cancer. Rev Endocr Metab Disord 15, 157–167 (2014). https://doi.org/10.1007/s11154-013-9265-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-013-9265-5