Abstract
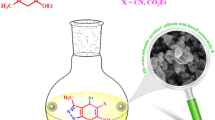
N-propyl sulfamic acid supported nano-catalyst on the basis of calix[4]resorcinarene was prepared via the efficient and facile reaction of amine functionalized polycalix[4]resorcinarene with chlorosulfonic acid. The achieved catalytic system was characterized using some spectroscopic techniques such as Fourier transform Infrared (FT-IR) spectroscopy, field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), and CHNS elemental analysis. This newly developed acidic catalyst was employed efficiently in a one-pot three-component condensation reaction of aromatic aldehydes, dimedone and phthalhydrazide for the synthesis of 2H-indazolo[2,1-b]phthalazine-trione derivatives through an easy and eco-friendly methodology. The catalyst was easily separated from the reaction mixture by simple filtration and the desired products were achieved in good to excellent yields in short reaction times.







Similar content being viewed by others
References
Anastas PT, Warner JC (1998) Green chemistry: theory and practice. Oxford University, Oxford
Zhang W, Cue B (2012) Green techniques for organic synthesis and medicinal chemistry. Wiley, Chichester
Mondloch JE, Bayram E, Finke RG (2012) A review of the kinetics and mechanisms of formation of supported-nanoparticle heterogeneous catalysts. J Mol Catal A 355:1–38
Dawson R, Cooper AI, Adams DJ (2012) Nanoporous organic polymer networks. Prog Polym Sci 37:530–563
Kaur P, Hupp JT, Nguyen ST (2011) Porous organic polymers in catalysis: opportunities and challenges. ACS Catal 1:819–835
Molla RA, Iqubal MA, Ghosh K, Kamaluddin, Islam SM (2015) Nitrogen enriched mesoporous organic polymer anchored copper(II) material: an efficient and reusable catalyst for the synthesis of esters and amides from aromatic systems. Dalton Trans 44:6546–6559
Choi DH, Ryoo R (2010) Template synthesis of ordered mesoporous organic polymeric materials using hydrophobic silylated KIT-6 mesoporous silica. J Mater Chem 20:5544–5550
Kundu SK, Bhaumik A (2015) Pyrene-based porous organic polymers as efficient catalytic support for the synthesis of biodiesels at room temperature. ACS Sustain Chem Eng 3:1715–1723
Li B, Guan Z, Yang X, Wang WD, Wang W, Hussain I, Song K, Tan B, Li T (2014) Multifunctional microporous organic polymers. J Mater Chem A 2:11930–11939
Suresh VM, Bonakala S, Atreya HS, Balasubramanian S, Maji TK (2014) Amide functionalized microporous organic polymer (Am-MOP) for selective CO2 sorption and catalysis. ACS Appl Mater Interfaces 6:4630–4637
Climent MJ, Corma A, Iborra S (2012) Homogeneous and heterogeneous catalysts for multicomponent reactions. RSC Adv 2:16–58
Zhang Q, Luo J, Wei Y (2010) A silica gel supported dual acidic ionic liquid: an efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkyl naphthols. Green Chem 12:2246–2254
Shaterian HR, Yarahmadi H, Ghashang M (2008) Silica supported perchloric acid (HClO4–SiO2): an efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkyl naphthols. Tetrahedron 64:1263–1269
Asif M (2012) Some recent approaches of biologically active substituted pyridazine and phthalazine drugs. Curr Med Chem 19:2984–2991
El-Sakka SS, Soliman AH, Imam AM (2009) Synthesis, antimicrobial activity and electron impact of mass spectra of phthalazine-1,4-dione derivatives. Afinidad 66:167–172
Singh S, Yadav A, Meena AK, Singh U, Singh B, Gaurav A, Rao MM, Panda P, Singh R (2010) Pharmacological action and SAR of phthalazine derivatives. Int J Chem Anal Sci 1:79–87
Ryu CK, Park RE, Ma MY, Nho JH (2007) Synthesis and antifungal activity of 6-arylamino-phthalazine-5,8-diones and 6,7-bis(arylthio)-phthalazine-5,8-diones. Bioorg Med Chem Lett 17:2577–2580
Sun XY, Wei CX, Deng XQ, Sun ZG, Quan ZS (2010) Evaluation of the anticonvulsant activity of 6-(4-chlorophenyoxy)-tetrazolo[5,1-a]phthalazine in various experimental seizure models in mice. Pharmacol Rep 62:273–277
Zhang L, Guan LP, Sun XY, Wei CX, Chai KY, Quan ZS (2009) Synthesis and anticonvulsant activity of 6-alkoxy-[1,2,4]triazolo[3,4-a] phthalazines. Chem Bio Drug Des 73:313–319
Li J, Zhao YF, Yuan XY, Xu JX, Gong P (2006) Synthesis and anticancer activities of novel 1,4-disubstituted phthalazines. Molecules 11:574–582
Watanabe N, Kabasawa Y, Takase Y, Matsukura M, Miyazaki K, Ishihara H, Kodama K, Adachi H (1998) 4-Benzylamino-1-chloro-6-substituted phthalazines: synthesis and inhibitory activity toward phosphodiesterase. J Med Chem 41:3367–3372
Kidwai M, Jahan A, Chauhan R, Mishra N, Neeraj K (2012) Dodecylphosphonic acid (DPA): a highly efficient catalyst for the synthesis of 2H-indazolo[2,1-b]phthalazine-triones under solvent-free conditions. Tetrahedron Lett 53:1728–1731
Nagarapu L, Bantu R, Mereyala HB (2009) TMSCl-mediated one-pot, three-component synthesis of 2H-indazolo[2,1-b]phthalazine-triones. J Heterocycl Chem 46:728–731
Khurana JM, Magoo D (2009) Efficient one-pot syntheses of 2H-indazolo[2,1-b] phthalazine-triones by catalytic H2SO4 in water–ethanol or ionic liquid. Tetrahedron Lett 50:7300–7303
Wang HJ, Zhang XN, Zhang ZH (2010) Highly efficient three-component synthesis of 1H-indazolo[1,2-b]phthalazinetrione derivatives catalyzed by heteropolyacids. Monatsh Chem 141:425–430
Hasaninejed A, Kazerooni MR, Zare A (2012) Solvent-free, one-pot, four-component synthesis of 2H-indazolo[2,1-b]phthalazine-triones using sulfuric acid-modified PEG-6000 as a green recyclable and biodegradable polymeric catalyst. Catal Today 196:148–155
Mosaddegh E, Hassankhani A (2011) A rapid, one-pot, four-component route to 2H-indazolo[2,1-b]phthalazine-triones. Tetrahedron Lett 52:488–490
Shaterian HR, Hosseinian A, Ghashang M (2009) Reusable silica supported poly phosphoric acid catalyzed three-component synthesis of 2H-indazolo[2,1-b]phthalazine-trione derivatives. Arkivoc 2:59–67
Shaterian HR, Ghashang M, Feyzi M (2008) Silica sulfuric acid as an efficient catalyst for the preparation of 2H-indazolo [2,1-b] phthalazine-triones. Appl Catal A 345:128–133
Sabitha G, Srinivas C, Raghavendar A, Yadav JS (2010) Phosphomolybdic acid (PMA)–SiO2 as a heterogeneous solid acid catalyst for the one-pot synthesis of 2H-Indazolo[1,2-b]phthalazine-triones. Helv Chim Acta 93:1375–1380
Sayyafi M, Seyyedhamzeh M, Khavasi HR, Bazgir A (2008) One-pot, three-component route to 2H-indazolo [2,1-b] phthalazine-triones. Tetrahedron 64:2375–2378
Wang X, Ma WW, Wu LQ, Yan FL (2010) Synthesis of 2H-Indazolo[2,1-b] phthalazine- 1,6,11(13H)-trione derivatives using wet cyanuric chloride under solvent-free condition. J Chin Chem Soc 57:1341–1345
Hamidian H, Fozooni S, Hassankhani A, Mohammadi SZ (2011) One-pot and efficient synthesis of triazolo[1,2-a]indazole-triones via reaction of arylaldehydes with urazole and dimedone catalyzed by silica nanoparticles prepared from rice husk. Molecules 16:9041–9048
Mouradzadegun A, Kiasat AR, Kazemian Fard P (2012) 3D-network porous polymer based on calix[4] resorcinarenes as an efficient phase transfer catalyst in regioselective conversion of epoxides to azidohydrins. Catal Commun 29:1–5
Mouradzadegun A, Ghasem Hezave F, Karimnia M (2010) Reductive alkylation of pentaphenylthiopyrylium perchlorate: an approac to regiospecific synthesis of hexasubstituted 2H-thiopyrans. Phosphorus Sulfur Silicon Relat Elem 185:84–87
Kiasat AR, Mouradzadegun A, Elahi S, Fallah-Mehrjardi M (2010) Al(HSO4)3/silica gel as a novel catalytic system for the ring opening of epoxides with thiocyanate anion under solvent-free conditions. Chin Chem Lett 21:146–150
Mouradzadegun A, Abadast F (2013) An improved, safe, and efficient conversion of triarylpyrylium perchlorates to corresponding cyanodienones using amberlite. Monatsh Chem 144:375–379
Mouradzadegun A, Mostafavi MA (2016) Copper-loaded hypercrosslinked polymer decorated with pendant amine groups: a green and retrievable catalytic system for quick [3 + 2] Huisgen cycloaddition in water. RSC Adv 6:42522–42531
Mouradzadegun A, Gheitasvand N (2005) Efficient reduction of thiopyrylium salts to corresponding 2H- and 4H-thiopyrans under solvent-free condition: regioselectivity and mechanism. Phosphorus Sulfur Silicon Relat Elem 180:1385–1388
Mouradzadegun A, Abadast F (2013) Thermally-induced ring contraction as a novel and straightforward route for the synthesis of 2-furyl acetonitrile derivatives. Tetrahedron Lett 54:2641–2644
Mouradzadegun A, Elahi S, Abadast F (2014) One-pot synthesis of tweezer-like calix[4]resorcinarene decorated with pendant heterocyclic moieties: an efficient and recyclable heterogeneous ptc for the preparation of azidohydrins in water. Catal Lett 144:1636–1641
Mouradzadegun A, Elahi S, Abadast F (2014) Synthesis of a 3D-network polymer supported Bronsted acid ionic liquid based on calix[4]-resorcinarene via two post-functionalization steps: a highly efficient and recyclable acid catalyst for the preparation of symmetrical bisamides. RSC Adv 4:31239–31248
Mouradzadegun A, Abadast F (2014) An improved organic/inorganic solid receptor for colorimetric cyanide-chemosensing in water: towards new mechanism aspects, simplistic use and portability. Chem Commun 50:15983–15986
Mouradzadegun A, Dianat S (2009) Facile and selective solvent-free synthesis of 2-isoxazolines under microwave irradiation. J Heterocycl Chem 46:778–781
Tunstad LM, Tucker JA, Dalcanale E, Weiser J, Bryant JA, Sherman JC, Helgeson RC, Knobler CB, Cram DJ (1989) Host-guest complexation. 48. Octal building blocks for cavitands and carcerands. J Org Chem 54:1305–1312
Altshuler H, Ostapova E, Fedyaeva O, Sapozhnikova L, Altshuler O (2002) Novel network polymers based on calixresorcinarenes. Macromol Symp 181:1–5
Karimi B, Zareyee D (2008) Design of a highly efficient and water-tolerant sulfonic acid nanoreactor based on tunable ordered porous silica for the von Pechmann reaction. Org Lett 10:3989–3992
Saha M, Phukan S, Jamatia R, Mitra S, Pal AK (2013) Solvent free, Ni-nanoparticle catalyzed greener synthesis and photophysical studies of novel 2H-indazolo[2,1-b] phthalazine-trione derivatives. RSC Adv 3:1714–1721
Shukla G, Verma RK, Verma GK, Singh MS (2011) Solvent-free sonochemical one-pot three-component synthesis of 2H-indazolo [2,1-b] phthalazine-1, 6, 11-triones and 1H-pyrazolo [1,2-b] phthalazine-5, 10-diones. Tetrahedron Lett 52:7195–7198
Mirhosseini-Eshkevari B, Ghasemzadeh MA, Safaei-Ghomi J (2015) An efficient and green one-pot synthesis of indazolo[1,2-b]-phthalazinetriones via three-component reaction of aldehydes, dimedone, and phthalhydrazide using Fe3O4@SiO2 core-shell nanoparticles. Res Chem Intermed 41:7703–7714
Godajdar BM, Kiasat AR, Hashemi MM (2013) One-pot synthesis of 2H-indazolo [2,1-b] phthalazinetrione catalyzed by magnetic room temperature dicationic ionic liquid under solvent-free conditions. Heterocycles 87:559–570
Alinasab Amiri A, Javanshir S, Dolatkhah Z, Dekamin MG (2015) SO3H-functionalized mesoporous silica materials as solid acid catalyst for facile and solvent-free synthesis of 2H-indazolo[2,1-b]phthalazine-1,6,11-trione derivatives. New J Chem 39:9665–9671
Acknowledgements
This work was supported by the Research Council at the University of Shahid Chamran.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mouradzadegun, A., Mostafavi, M.A. & Ganjali, M.R. A novel sulfamic acid functionalized nano-catalyst on the basis of calix[4]resorcinarene for the green one-pot synthesis of 2H-indazolo[2,1-b]phthalazine-triones under thermal solvent-free conditions. Reac Kinet Mech Cat 124, 741–755 (2018). https://doi.org/10.1007/s11144-018-1363-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-018-1363-7