Abstract
Purpose
Nighttime salivary cortisol (NSC) has been proposed for the diagnosis of Cushing’s syndrome during pregnancy. However, reference values for NCS in pregnant women have not been adequately determined. The aim of this study was to determine the reference values of NSC in the three gestational trimesters in order to help distinguish physiological from pathological hypercortisolism during pregnancy.
Methods
This prospective and retrospective study evaluated 85 pregnant women in whom samples were collected in the first, second and/or third gestational trimester (pregnancy group), 33 non-pregnant women (control group), and 25 non-pregnant women with Cushing’s disease (CD group). NSC was measured by enzyme-linked immunosorbent assay.
Results
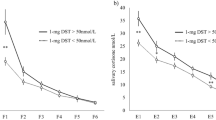
NSC increased progressively during pregnancy, reaching maximum levels on the third trimester (median 2.1-fold increase compared with controls, p < 0.001). Reference values for NSC were determined and the upper limits on each gestational trimester were: first trimester 0.25 µg/dL (6.9 nmol/L), second trimester 0.26 µg/dL (7.2 nmol/L), and third trimester 0.33 µg/dL (9.1 nmol/L). Cutoff values that separated the CD group from the three trimesters in the pregnancy groups were, respectively, 0.255 µg/dL (7.0 nmol/L), 0.260 µg/dL (7.2 nmol/L), and 0.285 µg/dL (7.9 nmol/L). Comparison of NSC cutoff values in pregnant women with CD patients showed high sensitivity and specificity in all three trimesters.
Conclusions
We established cutoff values for determination of NSC which can be useful for pregnant women with a diagnostic suspicion of CD.




Similar content being viewed by others
References
Lindsay JR, Nieman LK (2005) The hypothalamic-pituitary-adrenal axis in pregnancy: challenges in disease detection and treatment. Endocr Rev 26(6):775–799
Tejura H, Weiner J, Gibby O, O’Leary AJ, Avasarala S (2005) Cushing’s syndrome in pregnancy. J Obstet Gynaecol 25(7):713–714
Karaca Z, Tanriverdi F, Unluhizarci K, Kelestimur F (2010) Pregnancy and pituitary disorders. Eur J Endocrinol 162(3):453–475
Vilar L, da Freitas MC, Lima LH, Lyra R, Kater CE (2007) Cushing’s syndrome in pregnancy: an overview. Arq Bras Endocrinol Metabol 51(8):1293–1302
Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Fava GA, Findling JW, Gaillard RC, Grossman AB, Kola B, Lacroix A, Mancini T, Mantero F, Newell-Price J, Nieman LK, Sonino N, Vance ML, Giustina A, Boscaro M (2003) Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 88(12):5593–5602
Castro M, Moreira AC (2007) Screening and diagnosis of Cushing’s syndrome. Arq Bras Endocrinol Metabol 51(8):1191–1198
Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM (2008) The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93(5):1526–1540
Carroll TB, Findling JW (2010) The diagnosis of Cushing’s syndrome. Rev Endocr Metab Disord 11(2):147–153
Lindsay JR, Jonklaas J, Oldfield EH, Nieman LK (2005) Cushing’s syndrome during pregnancy: personal experience and review of the literature. J Clin Endocrinol Metab 90(5):3077–3083
Kita M, Sakalidou M, Saratzis A, Ioannis S, Avramidis A (2007) Cushing’s syndrome in pregnancy: report of a case and review of the literature. Hormones (Athens) 6(3):242–246
Bronstein MD, Paraiba DB, Jallad RS (2011) Management of pituitary tumors in pregnancy. Nat Rev Endocrinol 7(5):301–310
Hampson E, Phillips SD, Soares CN, Steiner M (2013) Steroid concentrations in antepartum and postpartum saliva: normative values in women and correlations with serum. Biol Sex Differ 4(1):7
Scott EM, McGarrigle HH, Lachelin GC (1990) The increase in plasma and saliva cortisol levels in pregnancy is not due to the increase in corticosteroid-binding globulin levels. J Clin Endocrinol Metab 71(3):639–644
Viardot A, Huber P, Puder JJ, Zulewski H, Keller U, Muller B (2005) Reproducibility of nighttime salivary cortisol and its use in the diagnosis of hypercortisolism compared with urinary free cortisol and overnight dexamethasone suppression test. J Clin Endocrinol Metab 90(10):5730–5736
Manetti L, Rossi G, Grasso L, Raffaelli V, Scattina I, Del Sarto S, Cosottini M, Iannelli A, Gasperi M, Bogazzi F, Martino E (2013) Usefulness of salivary cortisol in the diagnosis of hypercortisolism: comparison with serum and urinary cortisol. Eur J Endocrinol 168(3):315–321
Ambroziak U, Kondracka A, Bartoszewicz Z, Krasnodezbska-Kiljanska M, Bednarczuk T (2015) The morning and late-night salivary cortisol ranges for healthy women may be used in pregnancy. Clin Endocrinol (Oxf) (2015). doi:10.1111/cen.12853. Published online 15 July 2015
Newell-Price J, Trainer P, Besser M, Grossman A (1998) The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev 19(5):647–672
Newell-Price J, Bertagna X, Grossman AB, Nieman LK (2006) Cushing’s syndrome. Lancet 367(9522):1605–1617
Bertagna X, Guignat L, Groussin L, Bertherat J (2009) Cushing’s disease. Best Pract Res Clin Endocrinol Metab 23(5):607–623
Nolten WE, Rueckert PA (1981) Elevated free cortisol index in pregnancy: possible regulatory mechanisms. Am J Obstet Gynecol 139(4):492–498
Cousins L, Rigg L, Hollingsworth D, Meis P, Halberg F, Brink G, Yen SS (1983) Qualitative and quantitative assessment of the circadian rhythm of cortisol in pregnancy. Am J Obstet Gynecol 145(4):411–416
Allolio B, Hoffmann J, Linton EA, Winkelmann W, Kusche M, Schulte HM (1990) Diurnal salivary cortisol patterns during pregnancy and after delivery: relationship to plasma corticotrophin-releasing-hormone. Clin Endocrinol (Oxf) 33(2):279–289
Jung C, Ho JT, Torpy DJ, Rogers A, Doogue M, Lewis JG, Czajko RJ, Inder WJ (2011) A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab 96(5):1533–1540
Ho JT, Lewis JG, O’Loughlin P, Bagley CJ, Romero R, Dekker GA, Torpy DJ (2007) Reduced maternal corticosteroid-binding globulin and cortisol levels in pre-eclampsia and gamete recipient pregnancies. Clin Endocrinol (Oxf) 66(6):869–877
Trainer PJ (2002) Corticosteroids and pregnancy. Semin Reprod Med 20(4):375–380
Murphy BE (1977) Conversion of cortisol to cortisone by the human uterus and its reversal in pregnancy. J Clin Endocrinol Metab 44(6):1214–1217
Baid SK, Sinaii N, Wade M, Rubino D, Nieman LK (2007) Radioimmunoassay and tandem mass spectrometry measurement of bedtime salivary cortisol levels: a comparison of assays to establish hypercortisolism. J Clin Endocrinol Metab 92(8):3102–3107
Liu H, Bravata DM, Cabaccan J, Raff H, Ryzen E (2005) Elevated late-night salivary cortisol levels in elderly male type 2 diabetic veterans. Clin Endocrinol (Oxf) 63(6):642–649
Carroll T, Raff H, Findling JW (2008) Late-night salivary cortisol measurement in the diagnosis of Cushing’s syndrome. Nat Clin Pract Endocrinol Metab 4(6):344–350
Aden U, Jung-Hoffmann C, Kuhl H (1998) A randomized cross-over study on various hormonal parameters of two triphasic oral contraceptives. Contraception 58(2):75–81
Wiegratz I, Kutschera E, Lee JH, Moore C, Mellinger U, Winkler UH, Kuhl H (2003) Effect of four different oral contraceptives on various sex hormones and serum-binding globulins. Contraception 67(1):25–32
Alexandraki KI, Grossman AB (2010) Novel insights in the diagnosis of Cushing’s syndrome. Neuroendocrinology 92(Suppl 1):35–43
Deutschbein T, Petersenn S (2013) Screening for Cushing’s syndrome: new immunoassays require adequate normative data. Horm Metab Res 45(2):118–123
Mert M, Tanakol R, Karpuzoglu H, Abbasoglu S, Yarman S, Boztepe H, Alagol F (2013) Spectral effect: each population must have its own normal midnight salivary cortisol reference values determined. Arch Med Sci 9(5):872–876
Acknowledgments
We would like to thank Milena Braga-Basaria, MD, for the intellectual input and translation of the manuscript into English and Jessica Kantrowitz for proofreading its final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no potential conflict of interest.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Human and Animal Rights and Informed Consent
The study was approved by the Ethics Committee for Analysis of Research Projects of the Clinical Board of Hospital das Clínicas of the School of Medicine, University of São Paulo. All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all participants included in the study.
Rights and permissions
About this article
Cite this article
Lopes, L.M.L., Francisco, R.P.V., Galletta, M.A.K. et al. Determination of nighttime salivary cortisol during pregnancy: comparison with values in non-pregnancy and Cushing’s disease. Pituitary 19, 30–38 (2016). https://doi.org/10.1007/s11102-015-0680-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-015-0680-3