Abstract
Purpose
To assess the long-term impact of postoperative two-field-conventional radiotherapy (RT) on neurocognitive functions of adult patients with operated pituitary adenomas (PA).
Methods
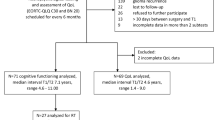
We selected 124 adult patients with operated PA—56 of whom had also received RT—recorded their main clinical data and performed a neuropsychological assessment in all of them that included 15 standardized tests, and a cerebral SPECT in eight patients. Comparative analyses were carried out on major clinical and neurocognitive domains between irradiated and not irradiated patients, and on cerebral SPECT source.
Results
Compared with non-irradiated patients, irradiated patients performed significantly worse on Barcelona’s story recall test (P < 0.001) and arithmetic problems (P < 0.03) and on five categories of the Wisconsin card sorting test, especially on perseverative answers and errors (P < 0.001) without differences in other examined functional domains. RT was the only factor associated with worse results in these tests regardless other clinical and treatment-related variables. Kaplan–Meier analysis suggested that the probability of achieving poorer results with time was related to RT total dose and field-size, type of PA and age at the time of RT. Four of the five SPECTS performed in irradiated patients revealed a similar altered perfusion in the left temporal lobe cortical region.
Conclusions
In adult patients with operated PA, RT was independently associated with an impairment on verbal memory and executive function, when compared to non-irradiated patients. Our data suggest that diagnosis of acromegaly or Cushing’s disease, and age at the time of RT were able to modulate this long-term radioinduced neurocognitive sequelae.


Similar content being viewed by others
References
Fischer AW, Holfelder H (1930) Lokales amyloid im gehirn. Dtsch Z Chir 227:475–483
Meadows AT, Gordon J, Massari DJ, Littman P, Fergusson J, Moss K (1981) Declines in IQ scores and cognitive dysfunctions in children with acute lymphocytic leukaemia treated with cranial irradiation. Lancet 2:1015–1018
Moore IM, Kramer JH, Wara W, Halberg F, Ablin AR (1991) Cognitive function in children with leukemia. Effect of radiation dose and time since irradiation. Cancer 68:1913–1917
Glosser G, McManus P, Munzenrider J, Austin-Seymour M, Fullerton B, Adams J, Urie MM (1997) Neuropsychological function in adults after high dose fractionated radiation therapy of skull base tumors. Int J Radiat Oncol Biol Phys 38:231–239
Abayomi OK (2002) Pathogenesis of cognitive decline following therapeutic irradiation for head and neck tumors. Acta Oncol 41:346–351
Redmond KJ, Mahone EM, Terezakis S, Ishaq O, Ford E, McNutt T, Kleinberg L, Cohen KJ, Wharam M, Horska A (2013) Association between radiation dose to neuronal progenitor cell niches and temporal lobes and performance on neuropsychological testing in children: a prospective study. Neuro-oncology 15:360–369
Gondi V, Hermann BP, Mehta MP, Tomé WA (2013) Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys 85:348–354
Schindler MK, Forbes ME, Robbins ME, Riddle DR (2008) Aging-dependent changes in the radiation response of the adult rat brain. Int J Radiat Oncol Biol Phys 70:826–834
Coderre JA, Morris GM, Micca PL, Hopewell JW, Verhagen I, Kleiboer BJ, van der Kogel AJ (2006) Late effects of radiation on the central nervous system: role of vascular endothelial damage and glial stem cell survival. Radiat Res 166:495–503
Monje ML, Mizumatsu S, Fike JR, Palmer TD (2002) Irradiation induces neural precursor-cell dysfunction. Nat Med 8:955–962
Ben Abdallah NM, Slomianka L, Lipp HP (2007) Reversible effect of X-irradiation on proliferation, neurogenesis, and cell death in the dentate gyrus of adult mice. Hippocampus 17:1230–1240
Monje ML, Toda H, Palmer TD (2003) Inflammatory blockade restores adult hippocampal neurogenesis. Science 302:1760–1765
Yazlovitskaya EM, Edwards E, Thotala D, Fu A, Osusky KL, Whetsell WO Jr, Boone B, Shinohara ET, Hallahan DE (2006) Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Res 66:11179–11186
Luria AR (1976) The neuropsycology of memory. John Wiley, New York
Lishman WA (1987) Organic psychiatry: the psychological consecuences of cerebral disorders. Blackwell, Oxford
Starkman MN, Schteingart DE (1981) Neuropsychiatric manifestifications of patients with Cushing’s syndrome. Arch Intern Med 141:215–219
Dorn LD, Burgess ES, Dubbert B, Simpson SE, Friedman T, Kling M, Gold PW, Chrousos GP (1995) Psychopathology in patients with endogenous Cushing’s syndrome: atypical or melancholic features. Clin Endocrinol 43:433–442
Leon-Carrion J, Martin-Rodriguez JF, Madrazo-Atutxa A, Soto-Moreno A, Venegas-Moreno E, Torres-Vela E, Benito-López P, Gálvez MA, Tinahones FJ, Leal-Cerro A (2010) Evidence of cognitive and neurophysiological impairment in patients with untreated naive acromegaly. J Clin Endocrinol Metab 95:4367–4379
Peace KA, Orme SM, Sebastian JP, Thompson AR, Barnes S, Ellis A, Belchetz PE (1997) The effect of treatment variables on mood and social adjustment in adult patients with pituitary disease. Clin Endocrinol 46:445–450
Page RC, Hammersley MS, Burke CW, Wass JA (1997) An account of the quality of life of patients after treatment for non-functioning pituitary tumours. Clin Endocrinol 46:401–406
Grattan-Smith PJ, Morris JG, Shores EA, Batchelor J, Sparks RS (1992) Neuropsychological abnormalities in patients with pituitary tumours. Acta Neurol Scand 86:626–631
McCord MW, Buatti JM, Fennell EM, Mendenhall WM, Marcus RB Jr, Rhoton AL, Grant MB, Friedman WA (1997) Radiotherapy for pituitary adenoma: long-term outcome and sequelae. Int J Radiat Oncol Biol Phys 39:437–444
Peace KA, Orme SM, Padayatty SJ, Godfrey HP, Belchetz PE (1998) Cognitive dysfunction in patients with pituitary tumour who have been treated with transfrontal or transsphenoidal surgery or medication. Clin Endocrinol 49:391–396
Peace KA, Orme SM, Thompson AR, Padayatty S, Ellis AW, Belchetz PE (1997) Cognitive dysfunction in patients treated for pituitary tumours. J Clin Exp Neuropsychol 19:1–6
Guinan EM, Lowy C, Stanhope N, Lewis PD, Kopelman MD (1998) Cognitive effects of pituitary tumours and their treatments: two case studies and an investigation of 90 patients. J Neurol Neurosurg Psychiatry 65:870–876
Noad R, Narayanan KR, Howlett T, Lincoln NB, Page RC (2004) Evaluation of the effect of radiotherapy for pituitary tumours on cognitive function and quality of life. Clin Oncol 16:233–237
Tiemensma J, Kokshoorn NE, Biermasz NR, Keijser BJ, Wassenaar MJ, Middelkoop HA, Pereira AM, Romijn JA (2010) Subtle cognitive impairments in patients with long-term cure of Cushing’s disease. J Clin Endocrinol Metab 95:2699–2714
Tiemensma J, Biermasz NR, van der Mast RC, Wassenaar MJ, Middelkoop HA, Pereira AM, Romijn JA (2010) Increased psychopathology and maladaptive personality traits, but normal cognitive functioning, in patients after long-term cure of acromegaly. J Clin Endocrinol Metab 95:E392–E402
van Beek AP, van den Bergh AC, van den Berg LM, van den Berg G, Keers JC, Langendijk JA, Wolffenbuttel BH (2007) Radiotherapy is not associated with reduced quality of life and cognitive function in patients treated for nonfunctioning pituitary adenoma. Int J Radiat Oncol Biol Phys 68:986–991
Brummelman P, Sattler MG, Meiners LC, Elderson MF, Dullaart RP, van den Berg G, Koerts J, Tucha O, Wolffenbuttel BH, van den Bergh AC, van Beek AP (2012) Cognitive performance after postoperative pituitary radiotherapy: a dosimetric study of the hippocampus and the prefrontal cortex. Eur J Endocrinol 166:171–179
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Benton AL (1945) A visual retention test for clinical use. Arch Neurol Psychiatry 54:212–216
Nelson HE (1976) A modified card sorting test sensitive to frontal lobe defects. Cortex 12:313–324
Peña-Casanova J, Guardia J, Bertran-Serra I, Manero RM, Jarne A (1997) Shortened version of the Barcelona test (I): subtests and normal profiles. Neurologia 12:99–111
Aberg ND, Brywe KG, Isgaard J (2006) Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. Sci World J 6:53–80
Pruessner M, Pruessner JC, Hellhammer DH, Bruce Pike G, Lupien SJ (2007) The associations among hippocampal volume, cortisol reactivity, and memory performance in healthy young men. Psychiatry Res 155:1–10
Klement J, Hubold C, Hallschmid M, Loeck C, Oltmanns KM, Lehnert H, Born J, Peters A (2009) Effects of glucose infusion on neuroendocrine and cognitive parameters in Addison disease. Metabolism 58:1825–1831
Tytherleigh MY, Vedhara K, Lightman SL (2004) Mineralocorticoid and glucocorticoid receptors and their differential effects on memory performance in people with Addison’s disease. Psychoneuroendocrinology 29:712–723
Beclere J (1909) The radiotherapeutic treatment of tumours of the hypophysis, gigantism and acromegaly. Arch Roentgen Ray 3:114
Brada M, Rajan B, Traish D, Ashley S, Holmes-Sellors PJ, Nussey S, Uttley D (1993) The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin Endocrinol 38:571–578
Estrada J, Boronat M, Mielgo M, Magallón R, Millan I, Díez S, Lucas T, Barceló B (1997) The long-term outcome of pituitary irradiation after unsuccessful transsphenoidal surgery in Cushing’s disease. N Engl J Med 336:172–177
Erfurth EM, Bülow B, Mikoczy Z, Svahn-Tapper G, Hagmar L (2001) Is there an increase in second brain tumours after surgery and irradiation for a pituitary tumour? Clin Endocrinol 55:613–616
Brada M, Ford D, Ashley S, Bliss JM, Crowley S, Mason M, Rajan B, Traish D (1992) Risk of second brain tumour after conservative surgery and radiotherapy for pituitary adenoma. BMJ 304:1343–1346
Millar JL, Spry NA, Lamb DS, Delahunt J (1991) Blindness in patients after external beam irradiation for pituitary adenomas: two cases ocurring after small daily fractional doses. Clin Oncol 3:291–294
al-Mefty O, Kersh JE, Routh A, Smith RR (1990) The long-term side effects of radiation therapy for benign brain tumours in adults. J Neurosurg 73:502–512
Ayuk J, Stewart PM (2009) Mortality following pituitary radiotherapy. Pituitary 12:35–39
Burman P, Mattsson AF, Johannsson G, Höybye C, Holmer H, Dahlqvist P, Berinder K, Engström BE, Ekman B, Erfurth EM, Svensson J, Wahlberg J, Karlsson FA (2013) Deaths among adult patients with hypopituitarism: hypocortisolism during acute stress, and de novo malignant brain tumors contribute to an increased mortality. J Clin Endocrinol Metab 98:1466–1475
Sattler MG, van Beek AP, Wolffenbuttel BH, van den Berg G, Sluiter WJ, Langendijk JA, van den Bergh AC (2012) The incidence of second tumours and mortality in pituitary adenoma patients treated with postoperative radiotherapy versus surgery alone. Radiother Oncol 104:125–130
Sattler MG, Vroomen PC, Sluiter WJ, Schers HJ, van den Berg G, Langendijk JA, Wolffenbuttel BH, van den Bergh AC, van Beek AP (2013) Incidence, causative mechanisms, and anatomic localization of stroke in pituitary adenoma patients treated with postoperative radiation therapy versus surgery alone. Int J Radiat Oncol Biol Phys 87:53–59
Armstrong CL, Gyato K, Awadalla AW, Lustig R, Tochner ZA (2004) A critical review of the clinical effects of therapeutic irradiation damage to the brain: the roots of controversy. Neuropsychol Rev 14:65–86
Naylor AS, Bull C, Nilsson MK, Zhu C, Björk-Eriksson T, Eriksson PS, Blomgren K, Kuhn HG (2008) Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci USA 105:14632–14637
Ramón YCS (1952) Structure and connections of neurons. Bull Los Angel Neuro Soc 17:5–46
Morris RG, Anderson E, Lynch GS, Baudry M (1986) Selective impairment of learning and blockade of long-term potentiation by an N-methyl-d-aspartate receptor antagonist, AP5. Nature 319:774–776
Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39
Hallbergson AF, Gnatenco C, Peterson DA (2003) Neurogenesis and brain injury: managing a renewable resource for repair. J Clin Investig 112:1128–1133
Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H (2009) Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 323:1074–1077
Acknowledgments
We thank all patients for their collaborative participation in this study and Ana Ruiz, José Ortiz Berrocal, and Rosa Magallón for their help in selecting the battery of neurocognitive tests, SPECT performance and interpretation, and radiotherapy data collection, respectively.
Conflict of interest
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lecumberri, B., Estrada, J., García-Uría, J. et al. Neurocognitive long-term impact of two-field conventional radiotherapy in adult patients with operated pituitary adenomas. Pituitary 18, 782–795 (2015). https://doi.org/10.1007/s11102-015-0653-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-015-0653-6