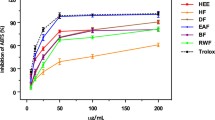
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity of the crude methanolic extract from the soft coral Sarcophyton flexuosum Tixier-Durivault was assayed. The crude methanolic extract of S. flexuosum collected from Lakshadweep Island showed significant potential in antioxidant activity. GC-MS analysis of the hexane and chloroform extracts of S. flexuosum revealed the presence of 27 compounds belonging to five major groups including esters, hydrocarbons, carbonyls, ethers, and alcohols. In these compounds, fatty acid esters were found to be the major chemical group, followed by hydrocarbons, ethers, carbonyls, and alcohols. Among the compounds identified, butyl butyrate, methyl palmitate, methyl (9E)-9-octadecenoate, propanoic acid butyl ester, undecane, 2-ethyl-hexan-1-ol and isosteviol were found to be pharmacologically important, based on their reported activity.



Similar content being viewed by others
References
A. M. S Mayer, and V. K. B Lehmann, Pharmacologist, 42, 62 – 69 (2000).
J. W. Blunt, R. Brent, C. Robert, et al., Natur. Prod. Rep., 28, 196 (2011).
D. J. Faulkner., Natur. Prod. Rep., 19, 1 – 43 (2002).
D. J. Faulkner and W. Fenical, Tetrahedron, 33, 1421 – 1443 (1977).
Y. Benayahu, and Y. Loya, Helgolander wiss. Meeresunters., 30, 362 – 382 (1977).
Y. Neeman, L. Fishelson, and Y. Kashman, Toxicon, 12, 593 – 598 (1974).
J. C. Coll., Chem. Rev., 92, 613 – 631 (1992).
O. Yamauchi, M. Omori, M. Ninomiya, et al., Jpn. J. Cancer Res., 82, 1234 – 1238 (1991).
F. A. Badria, A. N. Guirguis, and W. A. El-Naggar, Int. J. Pharm., 5, 284 – 287 (1997).
C. Y. Wang, M. Y. Geng, and H. S. Guan, Chin. J. New Drugs, 14, 278 – 282 (2005).
G. W. Zhang, X. Q. Ma, H. Kurihara, et al., Org. Lett., 7(6), 991 – 994 (2005).
A. Prakash, F. Rigelhof, and E. Miller, Medallion Laboratories: Analytical progress.
Kriengsak Thaipong, et al., J. Food Compos. Anal., 19, 669 – 675 (2006).
http: // www.pesticideinfo.org / Detail Chemical.jsp?Rec Id= PC42007
P. S. Praveen Kumar, S. Kumaravel, and C. Lalitha, Afr. J. Biochem. Res., 4, 191 – 195 (2010).
F. R. Yu, X. Z. Lian, H. Y. Guo, et al., J. Pharm. Sci., 3, 528 – 535 (2005).
O. G. Mironov, T. L. Shchekaturina, and I. M. Tsimbal., Marine Ecol. Prog. Ser., 5, 303 – 309 (1981).
Y. S. Yoo, J. Osaka City Med. Center, 34, 267 – 288 (1986).
European Commission (2002): Opinion of the Scientific Committee on Food on the Presence of Hypericin and Extracts of Hypericum Sp. in Flavorings and Other Food Ingredients with Flavoring Properties. SCF / CS / FLAV / FLAVOUR / 5 ADD1 Retrieved from http: // europa.eu.int / comm / food / fs / sc / scf / out113 en.pdf
B. L. Van Durren and B. M. Goldschmidt, J. Natl. Cancer Inst., 56, 1237 – 1242 (1976).
http: // www.thegoodscentscompany.com / data / rw1573931.html
Hazardous Substances Data Bank, Toxicology and Environmental Health Information Program, National Library of Medicine, Bethesda, MD (2004), retrieved from http: // toxnet.nlm. nih.gov / cgibin / sis / htmlgen?HSDB
Toxicological Evaluation of Certain Food Additives and Contaminants, WHO Food Additives Series, No. 32, Prepared by the 41st Meeting of the Joint FAO / WHO Expert Committee on Food Additives (JEFCA), WHO, Geneva (1993). ISBN: 92 – 4 – 166032 – 5, retrieved from http: // www.inchem.org / documents / jecfa / jecmono / v32je04.htm.
R. A. Scala and E. G. Burtis, Am. Ind. Hygiene Assoc. J., 34, 493 – 499 (1973).
http: // www.thegoodscentscompany.com / data / rw1590941.html
http: // www.thegoodscentscompany.com / data / rw1026181.html
E. Mosetting and W. R. Nes, J. Org. Chem., 20, 884 – 899 (1955).
V. A. Al’fonsov, G. A. Bakaleinik, A. T. Gubaidullin, et al., Russ. J. Gen. Chem., 68, 1735 – 1743 (1998).
R. Z. Musin, V. M. Babaev, I. Yu. Strobykina, et al., Russ. J. Gen. Chem., 79, 2377 – 2382 (2009).
Deyi Xu, Wenfeng Du, Lei Zhao, et al., Planta Med., 74, 816 – 821 (2008).
Acknowledgments
Authors thank Dr. P. A Thomas for his helped in the taxonomic identification of the organism S. flexuosum Tixier-Durivault. The financial support received from the Government of Kerala to establish a new centre, Inter University Centre for the Development of Marine Biotechnology (IUCDMB) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Byju, K., Anuradha, V., Rosmine, E. et al. DPPH Scavenging Property of Active Principles from Soft Coral Sarcophyton Flexuosum Tixier-Durivault. Pharm Chem J 49, 178–182 (2015). https://doi.org/10.1007/s11094-015-1249-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-015-1249-1