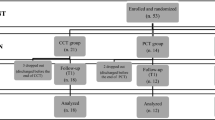
Objective. To run a parallel placebo-controlled trial to assess the effects of navigated combined high-frequency rhythmic transcranial magnetic stimulation (rTMS) of the primary motor (bilateral) and left dorsolateral prefrontal cortex on the clinical dynamics of the symptoms of Parkinson’s disease (PD). Materials and methods. A total of 46 patients took part in the trial and were randomized to active (n = 23) and placebo (n = 23) rTMS. Navigated therapeutic and placebo rTMS were performed for areas of the primary motor and left dorsolateral prefrontal cortex at a frequency of 10 Hz (20 daily sessions for three weeks). Changes in clinical symptoms were assessed on the MDS-UPDRS (parts I–IV) before sessions, immediately after 20 sessions, and 4–6 weeks after courses of rTMS. Nonmotor and mental symptoms were evaluated on the Hamilton depression scale (HDRS-17), the Beck scale (BDI-II), the depression, anxiety, and stress scale (DASS-21), and the mini mental state examination (MMSE). Results. Statistically signifi cant therapeutic effects were obtained with rTMS as compared with placebo, with greater reductions in total scores on the MDS-UPDRS (parts I–IV), the severity of nonmotor (part I)and motor (Part III, with greater therapeutic effects for rigidity, bradykinesia, and postural instability) signs, as well as the severity of motor complications of dopamine replacement therapy (part IV). The effects of rTMS on motor symptoms persisted at four weeks after completion of stimulation courses. It is also important to note that the signifi cant improvements in the rTMS and placebo groups were similar in terms of the magnitudes of reductions in the severity of daily motor symptoms (part II of the MDS-UPDRS) and increases in the total scores on the MMSE, HDRS, BDI-II, and DASS-21. Conclusions. Combined high-frequency rTMS of two areas of the cerebral cortex – the motor (bilaterally) and the left dorsolateral prefrontal – had positive therapeutic effects on the motor and affective symptoms of PD which were signifi cantly greater than obtained using placebo stimulation.
Similar content being viewed by others
References
T. Pringsheim, N. Jette, A. Frolkis, and T. D. Steeves, “The prevalence of Parkinson’s disease: A systematic review and meta-analysis,” Mov. Disord., 29, 1583–1590 (2014), https://doi.org/10.1002/mds.25945.
D. Aarsland, B. Creese, and K. R. Chaudhuri, “A new tool to identify patients with Parkinson’s disease at increased risk of dementia,” Lancet Neurol., 16, No. 8, 576–578 (2017), https://doi.org/https://doi.org/10.1016/S1474-4422(17)30170-9.
W. He, P. Y. Fong, T. W. H. Leung, and Y. Z. Huang, “Protocols of non-invasive brain stimulation for neuroplasticity induction,” Neurosci. Lett., 719, 133437 (2020), https://doi.org/10.1016/j.neulet.2018.02.045.
A. V. Chervyakov, A. Y. Chernyavsky, D. O. Sinitsyn, and M. A. Piradov, “Possible mechanisms underlying the therapeutic effects of transcranial magnetic stimulation,” Front. Hum. Neurosci., 9, 303 (2015), https://doi.org/10.3389/fnhum.2015.00303.
Z. Peng, C. Zhou, S. Xue, et al., “Mechanism of repetitive transcranial magnetic stimulation for depression,” Shanghai Arch. Psychiatry, 30, No. 2, 84–92 (2018), https://doi.org/10.11919/j.issn.1002-0829.217047.
B. Yulug, L. Hanoglu, E. Kilic, et al., “The neuroprotective role of repetitive transcranial magnetic stimulation (rTMS) for neurodegenerative diseases: A short review on experimental studies,” Mini. Rev. Med. Chem., 16, 1269 (2016), https://doi.org/10.2174/13895575166.66160523145154.
L. Dinkelbach, M. Brambilla, R. Manenti, and A. K. Brem, “Noninvasive brain stimulation in Parkinson’s disease: Exploiting crossroads of cognition and mood,” Neurosci. Biobehav. Rev., 75, 407–418 (2017), https://doi.org/10.1016/j.neubiorev.2017.01.021.
P. S. Boggio, F. Fregni, F. Bermpohl, et al., “Effect of repetitive TMS and fl uoxetine on cognitive function in patients with Parkinson’s disease and concurrent depression,” Mov. Disord., 20, No. 9, 1178–1184 (2005), https://doi.org/10.1002/mds.20508.
H. Kimura, M. Kurimura, K. Kurokawa, et al., “A comprehensive study of repetitive transcranial magnetic stimulation in Parkinson’s disease,” ISRN Neurology, 2011, 845453 (2011), https://doi.org/10.5402/2011/845453.
R. Manenti, M. Brambilla, A. Benussi, et al., “Mild cognitive impairment in Parkinson’s disease is improved by transcranial direct current stimulation combined with physical therapy,” Mov. Disord., 31, 715–724 (2016), https://doi.org/10.1002/mds.26561.
D. H. Benninger, K. Iseki, S. Kranick, et al., “Controlled study of 50-Hz repetitive transcranial magnetic stimulation for the treatment of Parkinson disease,” Neurorehabil. Neural Repair, 26, No. 9, 1096–1105 (2012), https://doi.org/10.1177/1545968312445636.
L. Cocchi, A. Zalesky, Z. Nott, et al., “Transcranial magnetic stimulation in obsessive-compulsive disorder: A focus on network mechanisms and state dependence,” NeuroImage Clin, 19, 661–674 (2018), https://doi.org/10.1016/j.nicl.2018.05.029.
J. P. Lefaucheur, N. André-Obadia, A. Antal, et al., “Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS),” Clin. Neurophysiol., 125, No. 11, 2150–2206 (2014), https://doi.org/10.1016/j.clinph.2014.05.021.
Y. Shirota, H. Ohtsu, M. Hamada, et al., “Supplementary motor area stimulation for Parkinson disease A randomized controlled study,” Neurology, 80, No. 15, 1400–1405 (2013), https://doi.org/10.1212/WNL.0b013e31828c2f66.
A. M. Goodwill, J. A. G. Lum, A. M. Hendy, et al., “Using non-invasive transcranial stimulation to improve motor and cognitive function in Parkinson’s disease: a systematic review and meta-analysis,” Sci. Rep., 7, 14840 (2017), https://doi.org/10.1038/s41598-017-13260-z.
T. Maruo, K. Hosomi, T. Shimokawa, et al., “High-frequency repetitive transcranial magnetic stimulation over the primary foot motor area in Parkinson’s disease,” Brain Stimul., 6, No. 6, 884–891 (2013), https://doi.org/10.1016/j.brs.2013.05.002.
A. C. Lanoue, G. J. Blatt, and J. J. Soghomonian, “Decreased parvalbumin mRNA expression in dorsolateral prefrontal cortex in Parkinson’s disease,” Brain Res., 1531, 37–47 (2013), https://doi.org/10.1016/j.brainres.2013.07.025.
C. Huang, C. Tang, A. Feigin, et al., “Changes in network activity with the progression of Parkinson’s disease,” Brain, 130, No. 07, 1834–1846 (2007), https://doi.org/10.1093/brain/awm086.
Y. Hosokai, Y. Nishio, K. Hirayama, et al., “Distinct patterns of regional cerebral glucose metabolism in Parkinson’s disease with and without mild cognitive impairment,” Mov. Disord., 24, No. 6, 854–862 (2009), https://doi.org/10.1002/mds.22444.
T. Hattori, S. Orimo, S. Aoki, et al., “Cognitive status correlates with white matter alteration in Parkinson’s disease,” Hum. Brain Mapp., 33, No. 3, 727–739 (2012), https://doi.org/10.1002/hbm.21245.
T. R. Melzer, R. Watts, M. R. MacAskill, et al., “Grey matter atrophy in cognitively impaired Parkinson’s disease,” J. Neurol. Neurosurg. Psychiatry, 83, No. 2, 188–194 (2012), https://doi.org/10.1136/jnnp-2011-300828.
E. Pal, F. Nagy, Z. Aschermann, et al., “The impact of left prefrontal repetitive transcranial magnetic stimulation on depression in Parkinson’s disease: A randomized, double-blind, placebo-controlled study,” Mov. Disord., 25, No. 14, 2311–2317 (2010), https://doi.org/10.1002/mds.23270.
M. Yokoe, T. Mano, T. Maruo, et al., “The optimal stimulation site for high-frequency repetitive transcranial magnetic stimulation in Parkinson’s disease: A double-blind crossover pilot study,” J. Clin. Neurosci., 47, 72–78 (2018), https://doi.org/10.1016/j.jocn.2017.09.023.
M. P. Lomarev, S. Kanchana, W. Bara-Jimenez, et al., “Placebocontrolled study of rTMS for the treatment of Parkinson’s disease,” Mov. Disord., 21, No. 3, 325–331 (2006), https://doi.org/10.1002/mds.20713.
M. Brys, M. D. Fox, S. Agarwal, et al., “Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: a randomized trial,” Neurology, 10, 1108–1212 (2016), https://doi.org/10.1212/WNL.0000000000003279.
Y. H. Chou, P. T. Hickey, M. Sundman, et al., “Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson disease: a systematic review and meta-analysis,” JAMA Neurol., 72, No. 4, 432–440 (2015), https://doi.org/10.1001/jamaneurol.2014.4380.
A. J. Hughes, S. E. Daniel, L. Kilford, and A. J. Lees, “Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases,” J. Neurol. Neurosurg. Psychiatry, 55, No. 3, 181–184 (1992), https://doi.org/https://doi.org/10.1136/jnnp.55.3.181.
M. M. Hoehn and M. D. Yahr, “Parkinsonism: onset, progression, and mortality,” Neurology, 17, No. 5, 427–442 (1967), https://doi.org/10.1212/01.wnl.0000405146.06300.91.
C. L. Tomlinson, R. Stowe, S. Patel, et al., “Systematic review of levodopa dose equivalency reporting in Parkinson’s disease,” Mov. Disord., 25, No. 15, 2649–2653 (2010), https://doi.org/10.1002/mds.23429.
C. G. Goetz, B. C. Tilley, S. R. Shaftman, et al. and the Movement Disorder Society UPDRS Revision Task Force, “Movement Disorder Society-sponsored revision of the Unifi ed Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results,” Mov. Disord., 23, No. 15, 2129–2170 (2008), https://doi.org/10.1002/mds.22340.
A. T. Beck, C. H. Ward, M. Mendelson, et al., “An inventory for measuring depression,” Arch. Gen. Psychiatry, 4, No. 6, 561–571 (1961), https://doi.org/10.1001/archpsyc.1961.01710120031004.
M. Hamilton, “A rating scale for depression,” J. Neurol. Neurosurg. Psychiatry, 23, No. 1, 56 (1960), https://doi.org/10.1136/jnnp.23.1.56.
P. F. Lovibond and S. H. Lovibond, “The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories,” Behav. Res. Ther., 33, No. 3, 335–343 (1995), https://doi.org/https://doi.org/10.1016/0005-7967(94)00075-U.
P. M. Rossini, D. Burke, R. Chen, et al., “Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee,” Clin. Neurophysiol., 126, No. 6, 1071–1107 (2015), https://doi.org/10.1016/j.clinph.2015.02.001.
A. V. Chervyakov, M. A. Piradov, M. A. Nazarova, et al., “Mapping of the motor representation of the abductor pollicis brevis in healthy volubnteers using navigated trnscranial magnetic stimulation with an NBS eXima Nexstim,” Ann. Klin. Eksperim. Nevrol., 6, No. 3, 14–17 (2012).
S. Groppa, A. Oliviero, A. Eisen, et al., “A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee,” Clin. Neurophysiol., 123, No. 5, 858–882 (2012), https://doi.org/10.1016/j.clinph.2012.01.010.
L. L. Gao and T. Wu, “The study of brain functional connectivity in Parkinson’s disease,” Transl. Neurodegener., 5, 18 (2016), https://doi.org/https://doi.org/10.1186/s40035-016-0066-0.
M. B. Ghabra, M. Hallett, and E. M. Wassermann, “Simultaneous repetitive transcranial magnetic stimulation does not speed fi ne movement in PD,” Neurology, 52, No. 4, 768–768 (1999), https://doi.org/10.1212/WNL.52.4.768.
F. Tergau, E. M. Wassermann, W. Paulus, and U. Ziemann, “Lack of clinical improvement in patients with Parkinson’s disease after low and high frequency repetitive transcranial magnetic stimulation,” Electroencephalogr. Clin. Neurophysiol., 51, 281–288 (1999).
S. Pallanti and A. Marras, “Transcranial magnetic stimulation treatment for motor symptoms in Parkinson’s disease: A review of two decades of studies,” J. Alzh. Dis. Parkinson., 5, 191 (2015), https://doi.org/https://doi.org/10.4172/2161-0460.1000191.
G. Koch, L. Brusa, C. Caltagirone, et al., “Subthalamic deep brain stimulation improves time perception in Parkinson’s disease,” NeuroReport, 15, No. 6, 1071–1073 (2004), https://doi.org/10.1097/00001756-200404290-00028.
D. H. Benninger, B. D. Berman, E. Houdayer, et al., “Intermittent theta-burst transcranial magnetic stimulation for treatment of Parkinson disease,” Neurology, 76, No. 7, 601–609 (2011), https://doi.org/10.1212/WNL.0b013e31820ce6bb.
I. Rektorova, S. Sedlackova, S. Telecka, et al., “Dorsolateral prefrontal cortex: A possible target for modulating dyskinesias in Parkinson’s disease by repetitive transcranial magnetic stimulation,” Int. J. Biomed. Imaging, 2008, 372125 (2008), https://doi.org/10.1155/2008/372125.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 120, No. 5, Iss. 1, pp. 29–36, May, 2020.
Rights and permissions
About this article
Cite this article
Aftanas, L.I., Brack, I.V., Kulikova, K.I. et al. Clinical and Neurophysiological Effects of the Therapeutic Combination of High-Frequency Rhythmic Transcranial Magnetic Stimulation of the Motor and Frontal Cortex in Parkinson’s Disease. Neurosci Behav Physi 51, 135–141 (2021). https://doi.org/10.1007/s11055-021-01048-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-021-01048-8