Abstract
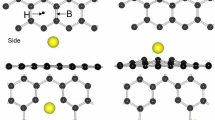
The adsorption of molecular NO on the free-standing and graphene-supported Mo3W5 cluster is studied using methods from the gradient-corrected density functional theory. Before, the effect of the graphene support on the properties of the metal cluster was investigated. The interaction between the metal cluster and the graphene sheet takes place mainly through W atoms, which form up to three bonds with the support. Interaction energies are in the range from 0.6 to 1.5 eV. An amount of charge of about 0.4–0.5 e\(^-\) is transferred from the cluster to the support. Geometric distortions in the metal aggregate are negligible. An important decrease in the magnetic moment of Mo3W5 with respect to its free-standing value is observed after the interaction with the support. Molecular NO adsorbs on sites involving W atoms only, both for the free-standing and the supported metal cluster. Adsorption energies are in a range from 2 to 4 eV. A parallel mode is the preferred mode from an energetic point of view. Moreover, for that parallel adsorption mode, the N–O bond is more effectively activated. Magnetic moments change largely after adsorption indicating important rearrangement in the electronic configuration of the metal cluster. An important amount of electronic charge is transferred both from the free-standing and from the supported metal cluster to NO. The amount of charge transferred seems to be closely related to the activation of the N–O bond. The effect of the graphene sheet on the catalytic properties of Mo3W5 seems to be negligible, with the exception of some changes in the electronic configuration of the cluster.





Similar content being viewed by others
References
Anderson ML, Ford MS, Derrik PJ, Drewello T, Woodruff DP, Mackenzie SR (2006) Nitric oxide decomposition on small rhodium clusters, Rhn +/-. J Phys Chem A 110:10992–11000
Brown WA, King DA (2000) NO chemisorption and reactions on metal surfaces: a new perspective. J Phys Chem B 104:2578–2578
Fan X, Zhang G, Zhang F (2015) Multiple roles of graphene in heterogeneous catalysis. Chem Soc Rev 44:3023–3035
Fogueri UR, Kozuch S, Karton A, Martin JM (2013) A simple DFT-based diagnostic for nondynamical correlation. Theor Chem Acc 132:1291–1295
Ford MS, Anderson ML, Barrow MP, Woodruff DP, Drewello T, Derrick PJ, Mackenzie SR (2005) Reactions of nitric oxide on Rh+ 6 clusters: abundant chemistry and evidence of structural isomers. Phys Chem Chem Phys 7:975–980
Harding D, Mackenzie SR, Walsh TR (2006) Structural isomers and reactivity for Rh6 and Rh+ 6. J Phys Chem B 110(18):272
Huber KP, Herzberg G (1979) Molecular spectra and molecular structure. IV. Constants of diatomic molecules, Van Nostrand Reinhold Co, Berkshire
Julkapli NM, Bagheri S (2015) Graphene supported heterogeneous catalysts: an overview. Int J Hydrog Energy 40:948–979
Kazusaka A, Howe RF (1980) Interaction of nitric oxide with supported chromium, molybdenum, and tungsten catalysts. J Catal 63:447–455
Kim G, Kawazoe Y, Lee KR (2012) Controlled catalytic properties of platinum clusters on strained graphene. J Phys Chem Lett 3:1989–1996
Kleinman L, Bylander DM (1982) Efficacious form for model pseudopotentials. Phys Rev Lett 48:1425–1428
Kong XK, Chen CL, Chen QW (2014) Doped graphene for metal-free catalysis. Chem Soc Rev 43:2841–2857
Mulliken RS (1955) Electronic population analysis on LCAOMO molecular wave functions. J Chem Phys 23:1833–1840
Parr RG, Yang W (1989) Density Functional theory of atoms and molecules. Oxford University Press, Oxford
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Pis-Diez R, Aguilera-Granja F (2013) Structural stability, chemical order and reactivity pattern of Mo p W q clusters, with p + q = 8. Eur Phys J D 67:251–263
Soler JM, Artacho E, Gale JD, García A, Junquera J, Ordejón P, Sánchez-Portal D (2002) The SIESTA method for ab initio order- N materials simulation. J Phys 14:2745–2779
Topsøe H, Clausen BS, Massoth FE (1996) Hydrotreating catalysis. In: Anderson JR, Boudart M (eds) Catalysis, catalysis-science and technology, vol 11. Springer, Berlin Heidelberg, p 1
Torres MB, Aguilera-Granja F, Balbás LC, Vega A (2011) Ab initio study of the adsorption of NO on the Rh+ 6 cluster. J Phys Chem A 115:8350–8360
Troullier N, Martins JL (1991) Efficient pseudopotentials for plane-wave calculations. Phys Rev B 43:1993–2006
Wang HT, Feng Q, Cheng YC, Yao YB, Wang QX, Li K, Schwingenschogl U, Zhang XX, Yang W (2013) Atomic bonding between metal and graphene. J Phys Chem C 117:4632–4638
Wu MC, Goodman DW (1994) Particulate Cu on ordered Al2O3: reactions with nitric oxide and carbon monoxide. J Phys Chem 98:9874–9881
Xu X, Goodman DW (1994) The effect of particle size on nitric oxide decomposition and reaction with carbon monoxide on palladium catalysts. Catal Lett 24:31–35
Xu X, Chen P, Goodman DW (1994) A comparative study of the coadsorption of carbon monoxide and nitric oxide on Pd(100), Pd(111), and silica-supported palladium particles with infrared reflection-absorption spectroscopy. J Phys Chem 98:9242–9246
Zhou MA, Zhang AH, Dai ZX, Feng YP, Zhang C (2010) Strain-enhanced stabilization and catalytic activity of metal nanoclusters on graphene. J Phys Chem C 114(16):16541–16546
Acknowledgments
The authors acknowledge J. Limón from the Computer Center of IF-UASLP, Mexico, for technical support during the calculations carried out in the present work. RPD is member of the Scientific Researcher Career of CONICET, Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguilera-Granja, F., Pis-Diez, R. Molecular adsorption of NO on free-standing and on graphene-supported Mo3W5 cluster: a density functional theory investigation. J Nanopart Res 18, 121 (2016). https://doi.org/10.1007/s11051-016-3421-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3421-2