Abstract
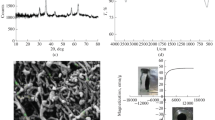
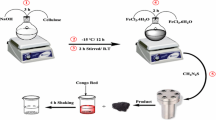
The removal of malachite green (MG) and rhodamine B (RB) from aqueous solutions was studied by batch adsorption technique using Fe3O4/MWCNT. Fe3O4/MWCNT adsorbent was synthesized by co-precipitation method and characterized by XRD, SEM, and TGA. 94 % MG removal and 98 % RB removal were achieved in 80 min using 10 mg adsorbent, at pH 6 and 298 K. The effects of adsorbent amount, pH, initial dye concentration, contact time, and temperature were investigated. Equilibrium data were well fitted by Langmuir adsorption model and pseudo-second-order kinetic model. MG adsorption on Fe3O4/MWCNT is exothermic and RB adsorption is endothermic in nature.












Similar content being viewed by others
References
Ahmad MA, Rahman NK (2011) Equilibrium, kinetics and thermodynamic of Remazol Brilliant Orange 3R dye adsorption on coffee husk-based activated carbon. Chem Eng J 170:154–161
Ai L, Huang H, Chen Z, Wei X, Jiang J (2010) Activated carbon/CoFe2O4 composites: facile synthesis, magnetic performance and their potential application for the removal of malachite green from water. Chem Eng J 156:243–249
Ai L, Zhang C, Liao F, Wang Y, Li M, Meng L, Jiang J (2011) Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: kinetic, isotherm and mechanism analysis. J Hazard Mater 198:282–290
Amin NK (2009) Removal of direct blue-106 dye from aqueous solution using new activated carbons developed from pomegranate peel: adsorption equilibrium and kinetics. J Hazard Mater 165:52–62
Anbia M, Hariri SA (2010) Removal of methylene blue from aqueous solution using nanoporous SBA-3. Desalination 261:61–66
Arellano-Cárdenas S, López-Cortez S, Cornejo-Mazón M, Mares-Gutiérrez JC (2013) Study of malachite green adsorption by organically modified clay using a batch method. Appl Surf Sci 280:74–78
Belaid KD, Kacha S, Kameche M, Derriche Z (2013) Adsorption kinetics of some textile dyes onto granular activated carbon. J Environ Chem Eng 1:496–503
Chen H, Zhao J, Wu J, Dai G (2011) Isotherm, thermodynamic, kinetics and adsorption mechanism studies of methyl orange by surfactant modified silkworm exuviae. J Hazard Mater 192:246–254
Doğan M, Özdemir Y, Alkan M (2007) Adsorption kinetics and mechanism of cationic methyl violet and methylene blue dyes onto sepiolite. Dyes Pigment 75:701–713
Faulconer EK, von Reitzenstein NVH, Mazyck DW (2012) Optimization of magnetic powdered activated carbon for aqueous Hg(II) removal and magnetic recovery. J Hazard Mater 199:9–14
Forgacs E, Cserhati T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971
Freundlich HMF (1906) Uber die adsorption in losungen. Z Phys Chem 57:385–471
Ghaedi M, Mosallanejad N (2014) Study of competitive adsorption of malachite green and sunset yellow dyes on cadmium hydroxide nanowires loaded on activated carbon. J Ind Eng Chem 20:1085–1096
Gonga J-L, Wang B, Zeng G-M, Yang C-P, Niu C-G, Niu Q-Y, Zhou W-J, Liang Y (2009) Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J Hazard Mater 164:1517–1522
Gupta VK, Kumar R, Nayak A, Saleh TA, Barakat MA (2013) Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv Colloid Interf Sci 193–194:24–34
Hong S, Wen C, He J, Gan F, Ho Y-S (2009) Adsorption thermodynamics of methylene blue onto bentonite. J Hazard Mater 167:630–633
Hou M-F, Mac C-X, Zhang W-D, Tang X-Y, Fan Y-N, Wan H-F (2011) Removal of rhodamine B using iron-pillared bentonite. J Hazard Mater 186:1118–1123
Huang J-H, Huang K-L, Liu S-Q, Wang A-T, Yan C (2008) Adsorption of Rhodamine B and methyl orange on a hypercrosslinked polymeric adsorbent in aqueous solution. Colloids Surf A 330:55–61
Jin X, Jiang M, Shan X, Pei Z, Chen Z (2008) Adsorption of methylene blue and orange II onto unmodified and surfactant-modified zeolite. J Colloid Interf Sci 328:243–247
Khan TA, Dahiya S, Ali I (2012) Use of kaolinite as adsorbent: equilibrium, dynamics and thermodynamic studies on the adsorption of Rhodamine B from aqueous solution. Appl Clay Sci 69:58–66
Khataee AR, Kasiri MB (2010) Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: influence of the chemical structure of dyes. J Mol Catal A 328:8–26
Kumar PS, Ramalingam S, Senthamarai C, Niranjanaa M, Vijayalakshmi P, Sivanesan S (2010) Adsorption of dye from aqueous solution by cashew nut shell: studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 261:52–60
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Li Y, Dua Q, Liu T, Peng X, Wang J, Sun J, Wang Y, Wu S, Wang Z, Xia Y, Xia L (2013) Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem Eng Res Des 91:361–368
Mandel K, Hutter F, Gellermann C, Sextl G (2013) Reusable superparamagnetic nanocomposite particles for magnetic separation of iron hydroxide precipitates to remove and recover heavy metal ions from aqueous solutions. Sep Purif Technol 109:144–147
Önal Y, Akmil-Başar C, Sarıcı-Özdemir Ç (2007) Investigation kinetics mechanisms of adsorption malachite green onto activated carbon. J Hazard Mater 146:194–203
Prola LDT, Machado FM, Bergmann CP, Souza FE, Gally CR, Lima EC, Adebayo MA, Dias SLP, Calvete T (2013) Adsorption of Direct Blue 53 dye from aqueous solutions by multi-walled carbon nanotubes and activated carbon. J Environ Manag 130:166–175
Qu S, Wang J, Kong J, Yang P, Chen G (2007) Magnetic loading of carbon nanotube/nano-Fe3O4 composite for electrochemical sensing. Talanta 71:1096–1102
Qu S, Huang F, Yu S, Chen G, Kong J (2008) Magnetic removal of dyes from aqueous solution using multi-walled carbon nanotubes filled with Fe2O3 particles. J Hazard Mater 160:643–647
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80
Rajeshwar K, Osugi ME, Chanmanee W, Chenthamarakshan CR, Zanoni MVB, Kajitvichyanukul P, Krishnan-Ayer R (2008) Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. J Photochem Photobiol C 9:171–192
Ren X, Chena C, Nagatsu M, Wang X (2011) Carbon nanotubes as adsorbents in environmental pollution management: a review. Chem Eng J 170:395–410
Shao L, Ren Z, Zhang G, Chen L (2012) Facile synthesis, characterization of a MnFe2O4/activated carbon magnetic composite and its effectiveness in tetracycline removal. Mater Chem Phys 135:16–24
Tian Y, Liu P, Wang X, Lin H (2011) Adsorption of malachite green from aqueous solutions onto ordered mesoporous carbons. Chem Eng J 171:1263–1269
Uma, Banerjee S, Sharma YC (2013) Equilibrium and kinetic studies for removal of malachite green from aqueous solution by a low cost activated carbon. J Ind Eng Chem 19:1099–1105
Wang S, Ng CW, Wang W, Li Q, Hao Z (2012) Synergistic and competitive adsorption of organic dyes on multiwalled carbon nanotubes. Chem Eng J 197:34–40
Zhang X, Li A, Jiang Z, Zhang Q (2006) Adsorption of dyes and phenol from water on resin adsorbents: effect of adsorbate size and pore size distribution. J Hazard Mater 137:1115–1122
Zhang L, He Y, Wu Y, Wu T (2011) Photocatalytic degradation of RhB over MgFe2O4/TiO2 composite materials. Mater Sci Eng B 176:1497–1504
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kerkez, Ö., Bayazit, Ş.S. Magnetite decorated multi-walled carbon nanotubes for removal of toxic dyes from aqueous solutions. J Nanopart Res 16, 2431 (2014). https://doi.org/10.1007/s11051-014-2431-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2431-1