Abstract
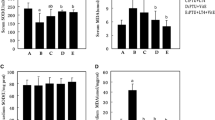
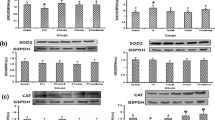
The present study was undertaken to investigate the effect of vitamin E and curcumin on the expression of antioxidant genes in 6-propyl-2-thiouracil (PTU)-induced hypothyroid rat renal cortex. The levels of lipid peroxidation and protein carbonylation were increased in hypothyroid rat kidney. Co-administration of vitamin E and curcumin to hypothyroid rats resulted in amelioration of lipid peroxidation level, whereas curcumin alone alleviated the protein carbonylation level. The mRNA levels of SOD1 and SOD2 were decreased in hypothyroid rats. Decreased level of SOD1 transcripts was observed in hypothyroid rats supplemented with curcumin alone or co-administrated with vitamin E. Translated products of SOD1 and SOD2 in hypothyroid rats was elevated in response to supplementation of both the antioxidants. Decreased SOD1 and SOD2 activities in hypothyroid rats compared to control were either unaltered or further decreased in response to the antioxidants. Expressions of CAT at transcript and translate level along with its activity were down regulated in hypothyroid rats. Administration of vitamin E to hypothyroid rats resulted in elevated CAT mRNA level. In contrast, expression of CAT protein was elevated in response to both the antioxidants. However, CAT activity was unaltered in response to vitamin E and curcumin. GPx1 and GR mRNA level and the activity of glutathione peroxidase (GPx) were not affected in response to induced hypothyroidism. The activity of GPx was increased in response to vitamin E treatment, whereas decreased GR activity in hypothyroid rats was further declined by the administration of antioxidants. The over all results suggest that vitamin E and curcumin differentially modulate the altered antioxidant defence mechanism of rat kidney cortex under experimental hypothyroidism.




Similar content being viewed by others
References
Mogulkoc R, Baltaci AK, Oztekin E, Aydin L, Tuncer I (2005) Hyperthyroidism causes lipid peroxidation in kidney and testis tissues of rats: protective role of melatonin. Neuro Endocrinol Lett 26:806–810
Mogulkoc R, Baltaci AK, Oztekin E, Ozturk A, Sivrikaya A (2005) Short-term thyroxine administration leads to lipid peroxidation in renal and testicular tissues of rats with hypothyroidism. Acta Biol Hung 56:225–232
Mogulkoc R, Baltaci AK, Oztekin E, Aydin L, Sivrikaya A (2006) Melatonin prevents oxidant damage in various tissues of rats with hyperthyroidism. Life Sci 13:311–315
Boveris A, Chance B (1973) The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134:707–716
Videla LA (2000) Energy metabolism, thyroid calorigenesis, and oxidative stress: functional and cytotoxic consequences. Redox Rep 5:265–275
Yen PM (2001) Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142
Venditti P, Di Meo S (2006) Thyroid hormone-induced oxidative stress. Cell Mol Life Sci 63:414–434
Venditti P, Balestrieri M, Di Meo S, De Leo T (1997) Effect of thyroid state on lipid peroxidation, antioxidant defences, and susceptibility to oxidative stress in rat tissues. J Endocrinol 155:151–157
Dumitriu L, Bartoc R, Ursu H, Purice M, Ionescu V (1988) Significance of high levels of serum malonaldehyde (MDA) and ceruloplasmin (CP) in hyper- and hypothyroidism. Endocrinologie 26:35–38
Yilmaz S, Ozan S, Benzer F, Canatan H (2003) Oxidative damage and antioxidant enzyme activities in experimental hypothyroidism. Cell Biochem Funct 21:325–330
Sies H (1991) Oxidative stress: from basic research to clinical application. Am J Med 91:31S–38S
Halliwell B, Gutteridge JMC (2001) In free radicals in biology and medicine, 3rd edn. Oxford University Press, New York
Kiya Y, Miura S, Zhang B, Urata H, Saku K (2006) Effect of levothyroxine on total lipid profiles as assessed by analytical capillary isotachophoresis in a patient with hypothyroidism. Endocrine J 53:865–868
Gerenova J, Gadjeva V (2007) Oxidative stress and antioxidant enzyme activities in patients with Hashimoto’s thyroiditis. Comp Clin Pathol 16:259–264. doi:10.1007/s00580-007-0689-8
Mogulkoc R, Baltaci AK, Aydin L, Oztekin E, Sivrikaya A (2005) The effect of thyroxine administration on lipid peroxidation in different tissues of rats with hypothyroidism. Acta Physiol Hung 92:39–46
Burton GW, Ingold KU (1989) Vitamin E as an in vitro and in vivo antioxidant. Ann NY Acad Sci 570:7–22
Michlin LJ, Bendich A (1987) Free radical tissue damage; protective role of antioxidant nutrients. FASEB J 1:441–445
Subudhi U, Das K, Paital B, Bhanja S, Chainy GBN (2009) Supplementation of curcumin and vitamin E enhances oxidative stress, but restores hepatic histoarchitecture in hypothyroid rats. Life Sci 84:372–379
Sahoo DK, Roy A, Chainy GBN (2008) Protective effects of vitamin E and curcumin on L-thyroxine-induced rat tesicular oxidative stress. Chem Biol Interact 176:121–128
Jena S, Chainy GBN (2010) Regulation of expression of antioxidant enzymes by vitamin E and curcumin in L-thyroxine-induced oxidative stress in rat renal cortex. Mol Biol Rep. doi:10.1007/s11033-010-0201-4
Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E, Dicato M, Diederich M (2005) Chemoprotective and therapeutic effects of curcumin. Cancer Lett 223:181–190
De Clercq E (2000) Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med Res Rev 20:323–349
Osawa T (2007) Nephroprotective and hepatoprotective effects of curcuminoids. Adv Exp Med Biol 595:407–423
Kaptein EM, Feinstein EI, Massry SG (1982) Thyroid hormone metabolism in renal diseases. Contribut Nephrol 33:122–135
den Hollander JG, Wulkan RW, Mantel MJ, Berghout A (2005) Correlation between severity of thyroid dysfunction and renal function. Clin Endocrinol (Oxford) 62:423–427
Ladenson PW, Kieffer JD, Farewell AP, Ridgway C (1986) Modulation of myocardial L-triidothyronine receptors in normal hypothyroid and hyperthyroid rats. Metabolism 35:5–12
Gabbianelli PW, Nasuti C, Falcioni G, Cantalamessa F (2004) Lymphocyte DNA damage in fats exposed to pyrethroids: effect of supplementation with vitamin E and C. Toxicology 203:17–26
Reddy ACP, Lokesh BR (1996) Effect of curcumin and eugenol on iron-induced hepatic toxicity in rats. Toxicology 107:39–45
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxide s in animal tissue by thiobarbituric acid reaction. Anal Biochem 95:352–358
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Mitsui A, Ohta T (1961) Photooxidative consumption and photoreproductive formation of ascorbic acid in green leaves. Plant Cell Physiol 2:31–44
Das K, Samanta L, Chainy GBN (2000) A modified spectrophotometric assay of superoxide dismutase using nitrite formation of superoxide radicals. Indian J Biochem Biophys 37:201–204
Okado-Matsumoto A, Fridovich I (2001) Subcellular distribution of superoxide dismutase (SOD) in rat liver: CuZn-SOD in mitochondria. J Biol Chem 276:38388–38393
Cohen G, Dembiec D, Marcus J (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34:30–38
Aebi H (1974) Catalase. In: Bergmayer HU (ed) Methods of enzymatic analysis, vol II. Academic press, New York, pp 673–683
Paglia DE, Valentine WN (1967) Studies on quantitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Massey V, Williams CH (1965) On the reaction mechanism of yeast glutathione reductase. J Biol Chem 240:4470–4481
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 377:239–242
Lindeman RD (1990) Overview: renal physiology and pathology of aging. Am J Kidney Dis 4:275–282
Montenegro J, Gonzalez O, Saracho R, Aguirre R, Gonzalez O, Martinez I (1996) Changes in renal function in primary hypothyroidism. Am J Kidney Dis 27:195–198
Radar DJ, Hobbs HH (2005) Disorders of lipoprotein metabolism. In: Kasper DL, Braunwald E, Fauei AS et al (eds) Harrison’s principle of internal medicine, vol II, 16th edn. McGraw-Hill, New York, p 2294
Ohara Y, Peterson TE, Harrison DG (1993) Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 91:2546–2551
Sutken E, Inal M, Ozdemir F (2006) Effects of vitamin E gemfibrozil on lipid profiles, lipid peroxidation and antioxidant status in the elderly and young hyperlipidemic subjects. Saudi Med J 27:453–459
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329:23–28
Banudevi S, Krishnamoorthy G, Venkataraman P, Vignesh C, Aruldhas MM, Arunakaran J (2006) Role of α-tocopherol on antioxidant status in liver, lung and kidney on PCB exposed male albino rats. Food Chem Toxicol 44:2040–2046
Patra RC, Swarup D, Dwivedi SK (2001) Antioxidants effects of α tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology 162:81–88
Tirkey N, Kaur G, Vij G, Chopra K (2005) Curcumin, a diferuloylmethane, attenutees cyclosporine-induced renal dysfunction and oxidative stress in rat kidneys. BMC Pharmacol. doi:10.1186/1471-2210-5-15
Rukkumani R, Aruna K, Varma PS, Rajasekaran KN, Menon VP (2004) Comparative effects of curcumin and an analog of curcumin on alcohol and PUFA induced oxidative stress. J Pharm Pharm Sci 7:274–283
Sies H (1999) Glutathione and its role in cellular functions. Free Radic Biol Med 27:916–921
Tas S, Dirican M, Sarandol E, Serdar Z (2006) The effect of taurine supplementation on oxidative stress in experimental hypothyroidism. Cell Biochem Funct 24:153–158
Asayama K, Dobashi K, Hayashibe H, Megata Y, Kato K (1987) Lipid peroxidation and free radical scavengers in thyroid dysfunction in the rat: a possible mechanism of injury to heart and skeletal muscle in hyperthyroidism. Endocrinology 121:2112–2118
Zheng S, Yumei F, Chen A (2007) De novo synthesis of glutathione is a prerequisite for curcumin to inhibit hepatic stellate cell (HSC) activation. Free Radic Biol Med 43:444–453
Pari L, Amali DR (2005) Protective role of tetrahydrocurcumin (THC) an active principle of turmeric on chloroquine induced hepatotoxicity in rats. J Pharm Pharm Sci 8:115–123
Luck MR, Jeyaseelan I, Scholes RA (1995) Ascorbic acid and fertility. Biol Reprod 52:262–266
Chung DJ, Wright AE, Clerch LB (1998) The 3′ untranslated region of manganese superoxide dismutase RNA contains a translated enhancer element. Biochemistry 37:30–38
Pasupathi P, Latha R (2008) Free radical activity and antioxidant defence mechanisms in patients with hypothyroidism. Thyroid Sci 3(12):1–6
Sen CK, Packer L (1996) Antioxidant and redox regulation of gene transcription. FASEB J 10:709–720
Strehlow K, Rotter S, Wassmann S, Adam O, Grohe C, Laufs K, Bohm M, Nickenig G (2003) Modulation of antioxidant enzyme expression and function by estrogen. Circ Res 93:170–177
Mayo JC, Sainz RM, Antoli I, Herrera F, Martin V, Rodriguez C (2002) Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci 59:1706–1713
Abe J, Berk BC (1999) Fyn and JAK2 mediate Ras activation by reactive oxygen species. J Biol Chem 274:21003–21010
Korontiz MI, Paplois A, Kouerinis I, Lazaris AC, Theodoropoulos G, Zografos G (2010) Redox state and potential antioxidant compounds in liver ischemia/reperfusion injury. Int J Med Med Sci 2:200–209
Pinkus R, Weiner LM, Daniel V (1996) Role of oxidants and antioxidants in the induction of AP-1, kappa B, and glutathione-S-transferase gene expression. J Biol Chem 271:13422–13429
Acknowledgments
This work was supported by Department of Biotechnology (DBT), Government of India. The 1st author is extremely grateful to the Department of Biotechnology, Government of India for providing the fellowship (Ref. No. DBT-JRF/05-06/123).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jena, S., Chainy, G.B.N. & Dandapat, J. Expression of antioxidant genes in renal cortex of PTU-induced hypothyroid rats: effect of vitamin E and curcumin. Mol Biol Rep 39, 1193–1203 (2012). https://doi.org/10.1007/s11033-011-0849-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-0849-4