Abstract
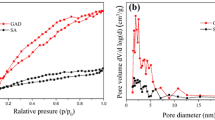
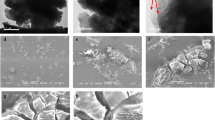
Graphene oxide was encapsulated into sodium alginate to prepare SA-GO composite hydrogel for U(VI) adsorption, which characterized by SEM, FTIR, and XPS. The effects of pH, reaction time, initial U(VI) concentration and adsorbent amount on U(VI) adsorption were studied by batch adsorption experiments. The results showed that the maximum adsorption capacity was 149.76 mg/L when pH 5.0, adsorbent 0.1 g/L, 30 °C and initial U(VI) concentration 15 mg/L. The adsorption process accords with pseudo-second-order kinetic models and Langmuir isotherm. After five cycles of desorption and reuse experiments, the adsorption capacity of SA-GO for U(VI) was still higher than 85%.










Similar content being viewed by others
References
Ouyang J, Liu Z, Ye T, Zhang L (2019) Uranium pollution status and speciation analysis in the farmLand-rice system around a uranium tailings mine in southeastern China. J Radioanal Nucl Ch 322(2):1011–1022
Bjørklund G, Semenova Y, Pivina L, Dadar M, Rahman MM, Aaseth J, Chirumbolo S (2020) Uranium in drinking water: a public health threat. Arch Toxicol 94(5):1551–1560
Bhalara PD, Punetha D, Balasubramanian K (2014) A review of potential remediation techniques for uranium(VI) ion retrieval from contaminated aqueous environment. J Environ Chem Eng 2(3):1621–1634
Wang Z, Hu H, Huang L, Lin F, Liu S, Wu T, Alharbi NS, Rabah SO, Lu Y, Wang X (2020) Graphene aerogel capsulated precipitants for high efficiency and rapid elimination of uranium from water. Chem Eng J 396:125272
Banala UK, Das NPI, Toleti SR (2020) Microbial interactions with uranium: Towards an effective bioremediation approach. Environ Technol Innovat 12:101254
Ren C, Kong C, Wang S, Xie Z (2019) Enhanced phytoremediation of uranium-contaminated soils by arbuscular mycorrhiza and rhizobium. Chemosphere 217:773–779
Khamirchi R, Hosseini-Bandegharaei A, Alahabadi A, Sivamani S, Rahmani-Sani A, Shahryari T, Anastopoulos I, Miri M, Tran HN (2018) Adsorption property of Br-PADAP-impregnated multiwall carbon nanotubes towards uranium and its performance in the selective separation and determination of uranium in different environmental samples. Ecotox Environ Safe 150:136–143
Negm SH, Abd El-Hamid AAM, Gado MA, El-Gendy HS (2019) Selective uranium adsorption using modified acrylamide resins. J Radioanal Nucl Ch 319(1):327–337
Li Z, Chen F, Yuan L, Liu Y, Zhao Y, Chai Z, Shi W (2012) Uranium(VI) adsorption on graphene oxide nanosheets from aqueous solutions. Chem Eng J 210:539–546
Qian Y, Yuan Y, Wang H, Liu H, Zhang J, Shi S, Guo Z, Wang N (208AD) Highly efficient uranium adsorption by salicylaldoxime/polydopamine graphene oxide nanocomposites. J Mater Chem A 6(48):24676–24685
Zaaba NI, Foo KL, Hashim U, Tan SJ, Liu W, Voon CH (2017) Synthesis of graphene oxide using modified hummers method: solvent influence. Proce Eng 184:469–477
Jiang X, Wang H, Wang Q, Hu E, Duan Y (2020) Immobilizing amino-functionalized mesoporous silica into sodium alginate for efficiently removing low concentrations of uranium. J Clean Prod 247:119162
Zhao C, Liu J, Deng Y, Tian Y, Zhang G, Liao J, Yang J, Yang Y, Liu N, Sun Q (2019) Uranium(VI) adsorption from aqueous solutions by microorganism-graphene oxide composites via an immobilization approach. J Clean Prod 236:117624
Zhuang Y, Yu F, Chen H, Zheng J, Ma J, Chen J (2016) Alginate/graphene double-network nanocomposite hydrogel beads with low-swelling, enhanced mechanical properties, and enhanced adsorption capacity. J Mater Chem A 4(28):10885–10892
Zeng J, Zhang H, Sui Y, Hu N, Ding D, Wang F, Xue J, Wang Y (2017) New amidoxime-based material TMP-g-AO for uranium adsorption under seawater conditions. Ind Eng Chem Res 56(17):5021–5032
Dou W, Yang W, Zhao X, Pan Q (2019) Hollow cobalt sulfide for highly efficient uranium adsorption from aqueous solutions. Inorg Chem Front 6(11):3230–3236
Khan MH, Warwick P, Evans N (2006) Spectrophotometric determination of uranium with arsenazo-III in perchloric acid. Chemosphere 63(7):1165–1169
Yu J, Wang J, Jiang Y (2017) Removal of uranium from aqueous solution by alginate beads. Nucl Eng Technol 49(3):534–540
Cheng H, Zeng K, Yu J (2013) Adsorption of uranium from aqueous solution by graphene oxide nanosheets supported on sepiolite. J Radioanal Nucl Ch 298(1):599–603
Viinikanoja A, Kauppila J, DamLin P, Suominen M, Kvarnström C (2015) In situ FTIR and Raman spectroelectrochemical characterization of graphene oxide upon electrochemical reduction in organic solvents. Phys Chem Chem Phys 17(18):12115–12123
Bai J, Fan F, Wu X, Tian W, Zhao L, Yin X, Fan F, Li Z, Tian L, Wang Y, Qin Z, Guo J (2013) Equilibrium, kinetic and thermodynamic studies of uranium biosorption by calcium alginate beads. J Environ Radioactiv 126:226–231
Fei Y, Li Y, Han S, Ma J (2016) Adsorptive removal of ciprofloxacin by sodium alginate/graphene oxide composite beads from aqueous solution. J Colloid Interf Sci 484:196–204
Wu F, Pu N, Ye G, Sun T, Wang Z, Song Y, Wang W, Huo X, Lu Y, Chen J (2017) Performance and mechanism of uranium adsorption from seawater to poly(dopamine)-inspired sorbents. Environ Sci Technol 51(8):4606–4614
Zhang A, Uchiyama G, Asakura T (2005) pH Effect on the uranium adsorption from seawater by a macroporous fibrous polymeric material containing amidoxime chelating functional group. React Funct Polym 63(2):143–153
Norrström AC, Löv Å (2014) Uranium theoretical speciation for drinking water from private drilled wells in Sweden – Implications for choice of removal method. Appl Geochem 51:148–154
Abdi S, Nasiri M, Mesbahi A, Khani MH (2017) Investigation of uranium (VI) adsorption by polypyrrole. J Hazard Mater 332:132–139
Huang Z, Li Z, Zheng L, Zhou L, Chai Z, Wang X, Shi W (2017) Interaction mechanism of uranium(VI) with three-dimensional graphene oxide-chitosan composite: Insights from batch experiments, IR, XPS, and EXAFS spectroscopy. Chem Eng J 328:1066–1074
Xue G, Yurun F, Li M, Dezhi G, Jie J, Jincheng Y, Haibin S, Hongyu G, Yujun Z (2017) Phosphoryl functionalized mesoporous silica for uranium adsorption. Appl Surf Sci 402:53–60
Nilchi A, Shariati Dehaghan T, Rasouli Garmarodi S (2013) Kinetics, isotherm and thermodynamics for uranium and thorium ions adsorption from aqueous solutions by crystalline tin oxide nanoparticles. Desalination 321:67–71
Liu B, Peng T, Sun H, Yue H (2017) Release behavior of uranium in uranium mill tailings under environmental conditions. J Environ Radioactiv 171:160–168
Kilislioglu A, Bilgin B (2003) Thermodynamic and kinetic investigations of uranium adsorption on amberlite IR-118H resin. Appl Radiat Isotopes 58(2):155–160
Chen B, Wang J, Kong L, Mai X, Zheng N, Zhong Q, Liang J, Chen D (2017) Adsorption of uranium from uranium mine contaminated water using phosphate rock apatite (PRA): Isotherm, kinetic and characterization studies. Colloids Surfaces A Physicochem Eng Aspects 520:612–621
Sureshkumar MK, Das D, Mallia MB, Gupta PC (2010) Adsorption of uranium from aqueous solution using chitosan-tripolyphosphate (CTPP) beads. J Hazard Mater 184(1):65–72
Zhuang S, Cheng R, Kang M, Wang J (2018) Kinetic and equilibrium of U(VI) adsorption onto magnetic amidoxime-functionalized chitosan beads. J Clean Prod 188:655–661
Yang A, Zhu Y, Huang CP (2018) Facile preparation and adsorption performance of graphene oxide-manganese oxide composite for uranium. Sci Rep-Uk 8(1):9058
Zong P, Wang S, Zhao Y, Wang H, Pan H, He C (2013) Synthesis and application of magnetic graphene/iron oxides composite for the removal of U(VI) from aqueous solutions. Chem Eng J 220:45–52
Tan L, Wang J, Liu Q, Sun Y, Jing X, Liu L, Liu J, Song D (2015) The synthesis of a manganese dioxide–iron oxide–graphene magnetic nanocomposite for enhanced uranium(vi) removal. New J Chem 39(2):868–876
Zhao Y, Li J, Zhao L, Zhang S, Huang Y, Wu X, Wang X (2014) Synthesis of amidoxime-functionalized Fe3O4@SiO2 core–shell magnetic microspheres for highly efficient sorption of U(VI). Chem Eng J 235:275–283
Wang CL, Li Y, Liu CL (2015) Sorption of uranium from aqueous solutions with graphene oxide. J Radioanal Nucl Ch 304(3):1017–1025
Li D, Hu N, Ding D, Li S, Li G, Wang Y (2016) An experimental study on the inhibitory effect of high concentration bicarbonate on the reduction of U(VI) in groundwater by functionalized indigenous microbial communities. J Radioanal Nucl Ch 307(2):1011–1019
Zhang H, Dai Z, Sui Y, Xue J, Ding D (2018) Adsorption of U(VI) from aqueous solution by magnetic core–dual shell Fe3O4@PDA@TiO2. J Radioanal Nucl Ch 317(1):613–624
Acknowledgements
This work was supported by the Educational Department of Guizhou Province (qian jiao he KY [2019]138;qian jiao he KY[2018]029), the Natural Science Foundation of Guizhou Province (qian ke he ji chu [2019]1293), and Liupanshui Normal University (LPSSYKYJJ201808).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, D., Zhang, P., Yang, Y. et al. U(VI) adsorption by sodium alginate/graphene oxide composite beads in water. J Radioanal Nucl Chem 327, 1131–1141 (2021). https://doi.org/10.1007/s10967-021-07598-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07598-y