Abstract
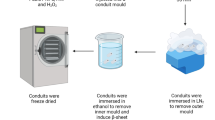
Peripheral nerves are exposed to physical injuries usually caused by trauma that may lead to a significant loss of sensory or motor functions and is considered as a serious health problem for societies today. This study was designed to develop a novel nano bioglass/gelatin conduit (BGGC) for the peripheral nerve regeneration. The bioglass nanoparticles were prepared by sol–gel technique and characterized using transmission electron microscopy (TEM), Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction analysis. The interfacial bonding interaction between the nano-bioglass and gelatin in the developed conduits was assessed by FTIR. The surface morphology and pore size of the nanocomposite were investigated through scanning electron microscopy with the pore size of the conduits being 10–40 μm. Biocompatibility was assessed by MTT assay which indicated the BGGC to have good cytocompatibility. The guidance channel was examined and used to regenerate a 10 mm gap in the right sciatic nerve of a male Wistar rat. Twenty rats were randomly divided into two experimental groups, one with the BGGC and the other being normal rats. The gastrocnemius muscle contractility was also examined at one, two and three months post-surgery in all groups using electromyography (EMAP). Histological and functional evaluation and the results obtained from electromyography indicated that at three months, nerve regeneration of the BGGC group was statistically equivalent to the normal group (p > 0.05). Our result suggests that the BGGC can be a suitable candidate for peripheral nerve repair.







Similar content being viewed by others
References
Moore MJ, Friedman JA, Lewellyn EB, Mantila SM, Krych AJ, Ameenuddin S, et al. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials. 2006;27:419–29.
Prang P, Muller R, Eljaouhari A, Heckmann K, Kunz W, Weber T, et al. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27:3560–9.
Amillo S, Yáñez R, Barrios RH. Nerve regeneration in different types of grafts: experimental study in rabbits. Microsurgery. 1995;16:621–30.
Balgude AP, Yu X, Szymanski A, Bellamkonda RV. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22:1077–84.
Chang JY, Lin JH, Yao CH, Chen JH, Lai TY, Chen YS. In vivo evaluation of a biodegradable EDC/NHS-cross-linked gelatin peripheral nerve guide conduit material. Macromol Biosci. 2007;7:500–7.
Pfister LA, Christen T, Merkle HP, Papaloïzos M, V B. Novel biodegradable. Novel biodegradable nerve conduits for peripheral nerve regeneration. Eur Cells and Mater. 2004;7:16–7.
Verreck G, Chun I, Li Y, Kataria R, Zhang Q, Rosenblatt J, et al. Preparation and physicochemical characterization of biodegradable nerve guides containing the nerve growth agent sabeluzole. Biomaterials. 2005;26:1307–15.
Azami M, Moztarzadeh F, Tahriri M. Preparation characterization and mechanical properties of controlled porous gelatin/hydroxyapatite nanocomposite through layer solvent casting combined with freeze-drying and lamination techniques. Porous Mater. 2009;17:313–20.
Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Biotechnology. 2005;23:74–5.
Lawrencin CT, Amin SFE, Ibim SE, Willoughby DA, Attavia M, Allcock HR, et al. A highly porous 3-dimensional polyphosphazene polymer matrix for skeletal tissue regeneration. J Biomed Mater Res. 1996;30:133–8.
Yuan H, De Bruijn JD, Zhang X, Bitterswijk CAV, De Groot K. Factors affecting the structure and properties of bioactive foam scaffolds for tissue engineering. J Biomed Mater Res. 2008;68:270–6.
Huang ZM, Zhang YZ, Ramakrishna S, Lim CT. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer. 2004;45:5361–8.
Jones JR, Ahir S, Hench LL. Large-scale production of 3D bioactive glass macroporous. Sol-Gel Sci Technol. 2004;29:179–88.
Taboas JM, Maddox RD, Krebsbach PH, Hollister SJ. Indirect solid free form fabrication of local and global porous, biomimetic and composite 3D polymer-ceramic scaffolds. Biomaterials. 2003;24:181–94.
Carrasquillo KG, Stanley AM, Aponte-Carro JC, De Jesus PDJ, Costantino HR, Bosques CJ. Non-aqueous encapsulation of excipient-stabilized spray freeze dried BSA into poly(lactide-co-glycolide) microspheres results in release of native protein. Control Release. 2001;76:199–208.
Fu H, Fu Q, Zhou N, Huang W, Rahaman MN, Wang D, et al. In vitro evaluation of borate-based bioactive glass scaffolds prepared by a polymer foam replication method. Eng Mater Sci Lett. 2009;29:2079–312.
Boland ED, Espy P, Bowlin GL. Tissue engineering scaffolds. In: Encyclopaedia of Biomaterials and biomedical engineering. 2004. p. 1633–5.
Boedtker H, Doty PA. A study of gelatin molecules aggregates and gels. J Phys Chem. 1954;58:968–83.
Gardin C, Ferroni L, Favero L, Stellini E, Stomaci D, Sivolella S, et al. Nanostructured biomaterials for tissue engineered bone tissue reconstruction. Int J Mol Sci. 2012;13:737–57.
Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–31.
Guarino V, Causa F, Ambrosio L. Bioactive scaffolds for bone and ligament tissue. Expert Rev Med Devices. 2007;4:405–18.
Liu X, Smith LA, Hu J, Ma PX. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials. 2009;30:2252–8.
Mozafari M, Moztarzadeh F, Rabiee M, Azami M, Maleknia S, Tahriri M, et al. Development of macroporous nanocomposite scaffolds of gelatin/bioactive glass prepared through layer solvent casting combined with lamination technique for bone tissue engineering. Ceram Int. 2010;36:2431–9.
Bunting S, Silvio LD, Deb S, Hall S. Bioresorbable glass fibres facilitate peripheral nerve regeneration. Hand Surgery. 2005;30:242–7.
Jones JR. Review of bioactive glass: from Hench to hybrids. Acta Biomater. 2013;9:4457–86.
Picot J. Human cell culture protocol. CA, USA; 2004.
Fassina L, Saino E, Visai L, Avanzini MA, Cusella De Angelis MG, Benazzo F, Van Vlierberghe S, Dubruel P, Magenes G. Use of a gelatin cryogel as biomaterial scaffold in the differentiation process of human bone marrow stromal cells. Conf Proc IEEE Eng Med Biol Soc. 2010;247–50. doi:10.1109/IEMBS.2010.5627475.
Hafezi F, Hosseinnejad F, Fooladi AA, Mohit Mafi S, Amiri A, Nourani MR. Transplantation of nano-bioglass/gelatin scaffold in a non-autogenous setting for bone regeneration in a rabbit ulna. J Mater Sci Mater Med. 2012;23:2783–92.
Eto M, Yoshikawa H, Fujimura H, Naba I, Sumi-Akamaru H, Takayasu S, et al. The role of CD36 in peripheral nerve remyelination after crush injury. Eur J Neurosci. 2003;17:2659–66.
Teng S, Shi J, Peng B, Chen FL. The effect of alginate addition on the structure and morphology of hydroxyapatite/gelatin Nanocomposites. Compos Sci Technol. 2006;66:1532–8.
Chang MC, Ko CC, Douglas WH. Conformational change of hydroxyapatite-gelatin nanocomposite by glutaraldehyde. Biomaterials. 2003;24:3087–94.
Minfang C, Junjun T, Yuying L, Debao L. Preparation of gelatin coated hydroxyapatite nanorods and the stability of its aqueous colloidal. Appl Surf Sci. 2008;254:2730–5.
Nojehdehian H, Moztarzadeh F, Baharvand H, Nazarian H, Tahriri M. Preparation and surface characterization of poly-l-lysine-coated PLGA microsphere scaffolds containing retinoic acid for nerve tissue engineering: in vitro study. Colloids Surf B Biointerfaces. 2009;73:23–9.
Bian YZ, Wang Y, Aibaidoula G, Chen GQ, Wu Q. Evaluation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) conduits for peripheral nerve regeneration. Biomaterials. 2009;30:217–25.
Chang C-J, Hsu S-H. The effect of high outflow permeability in asymmetric poly(dl-lactic acid-co-glycolic acid) conduits for peripheral nerve regeneration. Biomaterials. 2006;27:035–1042.
Ahmed MR, Vairamuthu S, Shafiuzama M, Basha SH, Jayakumar R. Microwave irradiated collagen tubes as a better matrix for peripheral nerve regeneration. Brain Res. 2005;1046:55–67.
Schnell E, Klinkhammer K, Balzer S, Gary B, Doris K, Paul D, et al. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28:3012–25.
Chen YS, Chang J, Cheng CY, Tsai FJ, Yao CH, Liu BS. An in vivo evaluation of a biodegradable genipin-cross-linked gelatin peripheral nerve guide conduit material. Biomaterials. 2005;26:3911–8.
Liu B, Cai SX, Ma KW, Xu ZL, Dai XZ, Yang L, Lin C, Fu XB, Sung KL, Li XK. Fabrication of a PLGA-collagen peripheral nerve scaffold and investigation of its sustained release property in vitro. J Mater Sci Mater Med. 2008;19:1127–32.
Evans JR, Brandt K, Katz S, Chauvin P, Otto L, Bogle M. Bioactive poly(l-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials. 2002;23:841–8.
Fathi MH, Mortzavi VA, Doostmohammadi A. Bioactive glass nanopowder for the treatment of oral bone defects. Dentistry. 2007;4:115–22.
Mami M, Lucas-Girot A, Oudadesse H, Dorbez-Sridi R, Mezahi F, Dietrich E, et al. Investigation of the surface reactivity of a sol gel derived glass in the ternary system SiO2-CaO-P2O5. Appl Surf Sci. 2008;254:7386–93.
Mortazavi V, Nahrkhalaji MM, Fathi MH, Mousavi SB, Esfahani BN. Antibacterial effects of sol-gel-derived bioactive glass nanoparticle on aerobic bacteria. Biomed Mater Res A. 2010;94:160–8.
Masoud M, Rabiei M, Azami M, Maleknia S. Biomimetic formation of apatite on the surface of porous gelatin/bioactive glass nanocomposite scaffolds. Appl Surf Sci. 2010;257:1740–9.
Yang Y, Zhao W, He J, Zhao Y, Ding F, Gu X. Nerve conduits based on immobilization of nerve growth factor onto modified chitosan by using genipin as a crosslinking agent. Pharm Biopharmaceutics. 2011;79:519–25.
Lu HH, El-Amin SF, Scott KD, Laurencin CT. Three-dimensional, bioactive, biodegradable, polymer-bioactive glass composite scaffolds with improved mechanical properties support collagen synthesis and mineralization of human osteoblast-like cells in vitro. Biomed Mater Res A. 2003;64:465–74.
Jantová S, Theiszová M, Matejov P, Bakoš D. Biocompatibility and cytotoxicity of bioglass-ceramic composite with various P2O5 content in Li2O-SiO2-CaO-CaF2-P2O5 system on fibroblast cell lines. Acta Chimica Slovaca. 2011;4:15–30.
Mligiliche NL, Tabata Y, Ide C. Nerve regeneration through biodegradable gelatin conduits in mice. East Afr Med J. 1999;76:400–6.
Yu W, Zhau W, Zhu C, Zhang X, Ye D, Zhang W, et al. Sciatic nerve regeneration in rats by a promising electrospun collagen/poly(ε-caprolactone) nerve conduit with tailored degradation rate. Neuroscience. 2011;12:1471–2202.
Peker F, Solakoglu C, Yuksel F, Kutlay M. Effects of time lapse on results of partial nerve injury repair. J Reconstr Microsurg. 2005;21:145–9.
Sinis N, Schulte-Eversum C, Doser M, Müller HW. Nerve regeneration across a 2-cm gap in the rat median nerve using a resorbable nerve conduit filled with Schwann cells. J Neurosurg. 2005;103:1067–76.
Acknowledgments
This work was supported by the grant from the Nano biotechnology research center of Baqiyatallah University of Medical Sciences and Iranian National Sciences foundation (INSF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koudehi, M.F., Fooladi, A.A.I., Mansoori, K. et al. Preparation and evaluation of novel nano-bioglass/gelatin conduit for peripheral nerve regeneration. J Mater Sci: Mater Med 25, 363–373 (2014). https://doi.org/10.1007/s10856-013-5076-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-013-5076-1