Abstract
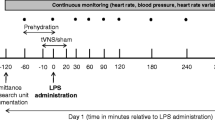
The cholinergic anti-inflammatory pathway has been identified as playing a key role in the communication between the central nervous system and the immune system during inflammation. The potential beneficial role of vagus nerve stimulation (VNS) remains to be clarified in established sepsis. We hypothesized that VNS or nicotine administration would reduce lung injury and mortality in established sepsis. We conducted a prospective, randomized experimental study. Four hours after peritonitis induction by cecal ligation and puncture (CLP), rats were randomized into three groups of seven animals according to the intervention: control group, VNS group (15 V, 2 ms, 5 Hz during 20 min), and nicotine group (400 µg/kg intraperitoneal). Survival was determined as lung injury score 4 and 8 h after CLP. Tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, IL-10, cytokine-induced neutrophil chemoattractant (CINC)-3 and thrombin–antithrombin complexes (TATc) were measured at baseline and at 4 and 8 h after CLP. Survival at 8 h was 71.4%, 100%, and 23.8% in the control, VNS, and nicotine groups, respectively (p < 0.05). All animals had lung damage but without significant difference between groups even if nicotine-treated animals tended to have a higher score than the controls (p = 0.09). Neutrophil polymorphonuclear (PMN) infiltration was more pronounced in the nicotine group compared with the VNS group (p = 0.015) but not with the controls. TNF-α, IL-6, IL-10, CINC-3, and TATc were elevated in all groups (NS). In this model of established sepsis, posttreatment by VNS was associated with increased survival, while nicotine administration increased lung PMN infiltration and mortality. Nicotine-induced bacterial clearance impairment and nicotine systemic effects may explain these observations.



Similar content being viewed by others
References
Dellinger, R.P. 2003. Inflammation and coagulation: Implications for the septic patient. Clinical Infectious Diseases 36: 1259–1265.
Levi, M., T. van der Poll, and H.R. Büller. 2004. Bidirectional relation between inflammation and coagulation. Circulation 109: 2698–2704.
Munford, R.S., and K.J. Tracey. 2002. Is severe sepsis a neuroendocrine disease? Molecular Medicine 8: 437–442.
Borovikova, L.V., S. Ivanova, M. Zhang, H. Yang, G.I. Botchkina, L.R. Watkins, H. Wang, N. Abumrad, J.W. Eaton, and K.J. Tracey. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462.
Wang, H., M. Yu, M. Ochani, C.A. Amella, M. Tanovic, S. Susarla, J.H. Li, H. Wang, H. Yang, L. Ulloa, Y. Al-Abed, C.J. Czura, and K.J. Tracey. 2003. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421: 384–388.
Saeed, R.W., S. Varma, T. Peng-Nemeroff, B. Sherry, D. Balakhaneh, J. Huston, K.J. Tracey, Y. Al-Abed, and C.N. Metz. 2005. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. Journal of Experimental Medicine 201: 1113–1123.
de Jonge, W.J., E.P. van der Zanden, F.O. The, M.F. Bijlsma, D.J. van Westerloo, R.J. Bennink, H.R. Berthoud, S. Uematsu, S. Akira, R.M. van den Wijngaard, and G.E. Boeckxstaens. 2005. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nature Immunology 6: 844–851.
Guarini, S., D. Altavilla, M.M. Cainazzo, D. Giuliani, A. Bigiani, H. Marini, G. Squadrito, L. Minutoli, A. Bertolini, R. Marini, E.B. Adamo, F.S. Venuti, and F. Squadrito. 2003. Efferent vagal fibre stimulation blunts nuclear factor-κB activation and protects against hypovolemic hemorrhagic shock. Circulation 107: 1189–1194.
Wang, H., H. Liao, M. Ochani, M. Justiniani, X. Lin, L. Yang, Y. Al-Abed, H. Wang, C. Metz, E.J. Miller, K.J. Tracey, and L. Ulloa. 2004. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nature Medicine 10: 1216–1221.
van Westerloo, D.J., I.A. Giebelen, S. Florquin, J. Daalhuisen, M.J. Bruno, A.F. de Vos, K.J. Tracey, and T. van der Poll. 2005. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. Journal of Experimental Medicine 191: 2138–2148.
van Westerloo, D.J., I.A. Giebelen, J.C. Meijers, J. Daalhuisen, A.F. de Vos, M. Levi, and T. van der Poll. 2006. Vagus nerve stimulation inhibits activation of coagulation and fibrinolysis during endotoxemia in rats. Journal of Thrombosis and Haemostasis 4: 1997–2002.
Hubbard, W.J., M. Choudhry, M.G. Schwacha, J.D. Kerby, L.W. Rue 3rd, K.I. Bland, and I.H. Chaudry. 2005. Cecal ligation and puncture. Shock 24(S1): 52–57.
Huston, J.M., M. Ochani, M. Rosas-Ballina, H. Liao, K. Ochani, V.A. Pavlov, M. Gallowitsch-Puerta, M. Ashok, C.J. Czura, B. Foxwell, K.J. Tracey, and L. Ulloa. 2006. Splenectomy inactivates the cholinergic anti-inflammatory pathway during lethal endotoxemia and polymicrobial sepsis. Journal of Experimental Medicine 203: 1623–1628.
Song, X.M., J.G. Li, Y.L. Wang, Z.F. Hu, Q. Zhou, Z.H. Du, and B.H. Jia. 2008. The protective effect of the cholinergic anti-inflammatory pathway against septic shock in rats. Shock 30: 468–472.
Hofer, S., C. Eisenbach, I.K. Lukic, L. Schneider, K. Bode, M. Brueckmann, S. Mautner, M.N. Wente, J. Encke, J. Werner, A.H. Dalpke, W. Stremmel, P.P. Nawroth, E. Martin, P.H. Krammer, A. Bierhaus, and M.A. Weigand. 2008. Pharmacologic cholinesterase inhibition improves survival in experimental sepsis. Critical Care Medicine 36: 404–408.
Huston, J.M., M. Gallowitsch-Puerta, M. Ochani, K. Ochani, R. Yuan, M. Rosas-Ballina, M. Ashok, R.S. Goldstein, S. Chavan, V.A. Pavlov, C.N. Metz, H. Yang, C.J. Czura, H. Wang, and K.J. Tracey. 2007. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Critical Care Medicine 35: 2762–2768.
Matsunaga, K., T.W. Klein, H. Friedman, and Y. Yamamoto. 2001. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. Journal of Immunology 167: 6518–6524.
Gillespie, M.N., J.O. Owasoyo, S. Kojima, and M. Jay. 1987. Enhanced chemotaxis and superoxide anion production by polymorphonuclear leukocytes from nicotine-treated and smoke-exposed rats. Toxicology 45: 45–52.
Sikora, L., S.P. Rao, and P. Sriramarao. 2003. Selectin-dependent rolling and adhesion of leukocytes in nicotine-exposed microvessels of lung allografts. American Journal of Physiology. Lung Cellular and Molecular Physiology 285: L654–L663.
Blum, B., and S. Zacks. 1958. Analysis of the relationship between drug-induced convulsions and mortality in rats. Journal of Pharmacology and Experimental Therapeutics 124: 350–356.
Acknowledgments
None.
Competing Interests
None.
Authors' Contributions
CB, VC, EL, and CL performed the study and data analysis. CB, XW, and PFL wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boland, C., Collet, V., Laterre, E. et al. Electrical Vagus Nerve Stimulation and Nicotine Effects in Peritonitis-Induced Acute Lung Injury in Rats. Inflammation 34, 29–35 (2011). https://doi.org/10.1007/s10753-010-9204-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-010-9204-5