Abstract
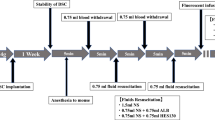
This study was designed to determine the effects of various resuscitation fluids on intestinal injuries after hemorrhagic shock and resuscitation (HS/R) and to determine the potential mechanisms. We induced HS by bleeding male Sprague-Dawley rats to a blood pressure of 30 to 40 mmHg for 60 min. Sixty minutes later, the rats were killed (HS group) or immediately resuscitated with L-isomer lactated Ringer’s solution (HS + LR group), shed blood (HS + BL group), or hydroxyethyl starch (HS + HES group) to maintain the blood pressure to the original value during the 60-min resuscitation period. Three hour after resuscitation, bacterial translocation (BT), intestinal permeability, ileal levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, malondialdehyde (MDA), oxidized and reduced glutathione (GSH and GSSG), myeloperoxidase (MPO) activity, nuclear factor (NF)-κB, activator protein (AP)-1 activation, and ileal microscopic and ultrastructural histological changes were measured. Another experiment was designed for survival study of 24 h. HES 130/0.4 solution was as effective as shed blood, required a small volume requirement to restore circulation, and significantly reduced HS/R-induced ileal villous morphological injuries with an anti-inflammatory effect, as reflected by a reduction of TNF-α, IL-6, MPO activity, and NF-κB activation. In addition, HES resuscitation also reduced intestinal permeability and BT and caused less oxidative stress as reflected by a reduction of MDA, GSSG/GSH and AP-1 activation along with restored GSH, whereas shed blood couldn’t. No significant difference was observed in outcome among groups. HES 130/0.4 resuscitation prevents HS/R induced intestinal injury by modulating inflammatory response and preventing oxidative stress in a rat model of hemorrhagic shock. These physiological protective effects appear to be mediated by down-regulation of the transcription factor NF-κB and AP-1.








Similar content being viewed by others
References
Lee, C. C., K. A. Marill, W. A. Carter, and R. S. Crupi. 2001. A current concept of trauma-induced multiorgan failure. Ann. Emerg. Med. 38:170–476 doi:10.1067/mem.2001.114313.
Lee, C. C., I. J. Chang, Z. S. Yen, C. Y. Hsu, S. Y. Chen, C. P. Su, W. C. Chiang, S. C. Chen, and W. J. Chen. 2007. Delayed fluid resuscitation in hemorrhagic shock induces proinflammatory cytokine response. Ann. Emerg. Med. 49(1):37–44 doi:10.1016/j.annemergmed.2006.05.031.
Tsai, M. C., W. J. Chen, C. H. Ching, and J. I. Chuang. 2007. Resuscitation with hydroxyethyl starch solution prevents nuclear factor kappaB activation and oxidative stress after hemorrhagic shock and resuscitation in rats. Shock. 27(5):527–33 doi:10.1097/01.shk.0000245032.31859.f2.
Imm, A., and R. W. Carlson. 1993. Fluid resuscitation in circulatory shock. Crit. Care. Clin. 9:313–333.
Deitch, E. A., J. Morrison, R. Berg, and R. D. Specian. 1990. Effect of hemorrhagic shock on bacterial translocation, intestinal morphology, and intestinal permeability in conventional and antibiotic-decontaminated rats. Crit. Care Med. 18:529 doi:10.1097/00003246-199005000-00014.
Tian, J., X. Lin, R. Guan, and J. G. Xu. 2004. The effects of hydroxyethyl starch on lung capillary permeability in endotoxic rats and possible mechanisms. Anesth. Analg. 98(3):768–774 doi:10.1213/01.ANE.0000099720.25581.86.
Lv, R., W. Zhou, L. D. Zhang, and J. G. Xu. 2005. Effects of hydroxyethyl starch on hepatic production of cytokines and activation of transcription factors in lipopolysaccharide-administered rats. Acta Anaesthesiol Scand. 49(5):635–642 doi:10.1111/j.1399-6576.2005.00668.x.
Feng, X., W. Yan, Z. Wang, J. Liu, M. Yu, S. Zhu, and J. Xu. 2007. Hydroxyethyl starch, but not modified fluid gelatin, affects inflammatory response in a rat model of polymicrobial sepsis with capillary leakage. Anesth. Analg. 104:624–630 doi:10.1213/01.ane.0000250366.48705.96.
Jungheinrich, C., W. Sauermann, F. Bepperling, and N. H. Vogt. 2004. Volume efficacy and reduced influence on measures of coagulation using hydroxyethyl starch 130/0.4 (6%) with an optimised in vivo molecular weight in orthopaedic surgery: a randomised, double-blind study. Drugs R D. 5:1–9 doi:10.2165/00126839-200405010-00001.
Wattanasirichaigoon, S., M. J. Menconi, R. L. Delude, and M. P. Fink. 1999. Effect of mesenteric ischemia and reperfusion or hemorrhagic shock on intestinal mucosal permeability and ATP content in rats. Shock. 12:127–133 doi:10.1097/00024382-199908000-00006.
Mihara, M., and M. Uchiyama. 1978. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 86:271–278 doi:10.1016/0003-2697(78)90342-1.
Chen, S. T., J. I. Chuang, M. H. Hong, and E. I. C. Li. 2002. Melatonin attenuates MPP+-induced neurodegeneration and glutathione impairment in nigrostriatal dopaminergic pathway. J. Pineal Res. 32:262–269 doi:10.1034/j.1600-079X.2002.01871.x.
Suzuki, K., H. Ota, S. Sasagawa, T. Sakatani, and T. Fujikura. 1983. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 132:345–352 doi:10.1016/0003-2697(83)90019-2.
Chiu, C. J., A. H. McArdle, R. Brown, H. J. Scott, and F. N. Gurd. 1970. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch. Surg. 101(4):478–483.
Kaszaki, J., A. Wolfárd, L. Szalay, and M. Boros. 2006. Pathophysiology of ischemia-reperfusion injury. Transplant Proc. 38(3):826–828 doi:10.1016/j.transproceed.2006.02.152.
Nemeth, I., and D. Boda. 1989. The ratio of oxidized/reduced glutathione as an index of oxidative stress in various experimental models of shock syndrome. Biomed. Biochim. Acta. 48:53–57.
Schnoor, J., R. Schreck, J. H. Baumert, C. Grosse-Siestrup, R. Rossaint, and J. K. Unger. 2004. Influence of differences in body weight and volume management on experimental results in porcine models. Int. J. Artif. Organs. 27:924–934.
Shi, H. P., E. A. Deitch, D. Z. Xu, Q. Lu, and C. J. Hauser. 2002. Hypertonic saline improves intestinal mucosal barrier function and lung injury after trauma-hemorrhagic shock. Shock. 17:496–501 doi:10.1097/00024382-200206000-00010.
Rhee, P., D. Burris, C. Kaufmann, et al. 1998. Lactated Ringer’s solution resuscitation causes neutrophil activation after hemorrhagic shock. J Trauma. 44:313–319 doi:10.1097/00005373-199802000-00014.
Ferreira, E. L., R. G. Terzi, W. A. Silva, and A. C. de Moraes. 2005. Early colloid replacement therapy in a near-fatal model of hemorrhagic shock. Anesth. Analg. 101(6):1785–1791 doi:10.1213/01.ANE.0000184133.48569.55.
Marx, G., M. Cobas Meyer, T. Schuerholz, B. Vangerow, K. F. Gratz, H. Hecker, R. Sumpelmann, H. Rueckoldt, and M. Leuwer. 2002. Hydroxyethyl starch and modified fluid gelatin maintain plasma volume in a porcine model of septic shock with capillary leakage. Intensive Care Med. 28:629–35 doi:10.1007/s00134-002-1260-3.
Taniguchi, T., Y. Koido, J. Aiboshi, T. Yamashita, S. Suzaki, and A. Kurokawa. 1999. The ratio of interleukin-6 to interleukin-10 correlates with severity in patients with chest and abdominal trauma. Am. J. Emerg. Med. 17:548–551 doi:10.1016/S0735-6757(99)90194-8.
Feng, X., J. Liu, M. Yu, S. Zhu, and J. Xu. 2007. Protective roles of hydroxyethyl starch 130/0.4 in intestinal inflammatory response and survival in rats challenged with polymicrobial sepsis. Clin. Chim. Acta. 376(1–2):60–67 doi:10.1016/j.cca.2006.07.008.
Fink, M. P. 2002. Reactive oxygen species as mediators of organ dysfunction caused by sepsis, acute respiratory distress syndrome, or hemorrhagic shock: potential benefits of resuscitation with Ringer’s ethyl pyruvate solution. Curr. Opin. Clin. Nutr. Metab. Care. 5:167–174 doi:10.1097/00075197-200203000-00009.
Granger, D. N., J. N. Benoit, M. Suzuki, and M. B. Grisham. 1989. Leukocyte adherence to venular endothelium during ischemia-reperfusion. Am. J. Physiol. 257:683–688.
Childs, E. W., K. F. Udobi, J. G. Wood, F. A. Hunter, D. M. Smalley, and L. Y. Cheung. 2002. In vivo visualization of reactive oxidants and leukocyte-endothelial adherence following hemorrhagic shock. Shock. 18:423–427 doi:10.1097/00024382-200211000-00006.
Sener, G., A. O. Sehirli, H. Satiroğlu, M. Keyer-Uysal, and B. C Yeğen. 2002. Melatonin improves oxidative organ damage in a rat model of thermal injury. Burns. 28(5):419–425 doi:10.1016/S0305-4179(02)00053-0.
Therade-Matharan, S., E. Laemmel, J. Duranteau, and E. Vicaut. 2004. Reoxygenation after hypoxia and glucose depletion causes reactive oxygen species production by mitochondria in HUVEC. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287:1037–1043 doi:10.1152/ajpregu.00048.2004.
Karabeyoğlu, M., B. Unal, B. Bozkurt, I. Dolapçi, A. Bilgihan, I. Karabeyoğlu, and O. Cengiz. 2008. The effect of ethyl pyruvate on oxidative stress in intestine and bacterial translocation after thermal injury. J. Surg. Res. 144(1):59–63 doi:10.1016/j.jss.2007.02.050.
Mori, T., H. Yamamoto, T. Tabata, T. Shimizu, Y. Endo, K. Hanasawa, M. Fujimiya, and T. Tani. 2005. A free radical scavenger, edaravone (MCI-186), diminishes intestinal neutrophil lipid peroxidation and bacterial translocation in a rat hemorrhagic shock model. Crit. Care. Med. 33(5):1064–1069 doi:10.1097/01.CCM.0000162952.14590.EC.
Vega, D., C. D. Badami, F. J. Caputo, A. C. Watkins, Q. Lu, Z. Xu da, T. L. Berezina, S. B. Zaets, E. Feketeova, and E. A. Deitch. 2008. The influence of the type of resuscitation fluid on gut injury and distant organ injury in a rat model of trauma/hemorrhagic shock. J. Trauma. 65(2):409–414 discussion 414–5.
Childs, E. W., J. G. Wood, D. M. Smalley, F. A. Hunter, and L. Y. Cheung. 1999. Leukocyte adherence and sequestration following hemorrhagic shock and total ischemia in rats. Shock. 11(4):248–252 doi:10.1097/00024382-199904000-00004.
Paxian, M., I. Bauer, D. Kaplan, M. Bauer, and H. Rensing. 2002. Hepatic redox regulation of transcription factors activator protein-1 and nuclear factor-kB after hemorrhagic shock in vivo. Antioxid Redox Signal. 4(5):711–20 doi:10.1089/152308602760598855.
Sha, W. C. 1998. Regulation of immune responses by NF-κB/Rel transcription factor. J. Exp. Med. 187:143–146 doi:10.1084/jem.187.2.143.
Altavilla, D., A. Saitta, G. Squadrito, M. Galeano, S. F. Venuti, S. Guarini, C. Bazzani, A. Bertolini, A. P. Caputi, and F. Squadrito. 2002. Evidence for a role of nuclear factor-kB in acute hypovolemic hemorrhagic shock. Surgery. 131:50–58 doi:10.1067/msy.2002.118320.
Sham, B. D., H. H. Barton, L. L. Reznikov, C. B. Cairns, A. Banerjee, A. H. Harken, and X. Meng. 2002. Ischemia alone is sufficient to induce TNF-alpha mRNA and peptide in the myocardium. Shock. 17:114–119 doi:10.1097/00024382-200202000-00006.
Rensing, H., H. Jaeschke, I. Bauer, C. Pätau, V. Datene, B. H. Pannen, and M. Bauer. 2001. Differential activation pattern of redox-sensitive transcription factors and stress-inducible dilator systems heme oxygenase-1 and inducible nitric oxide synthase in hemorrhagic and endotoxic shock. Crit Care Med. 29(10):1962–1971 doi:10.1097/00003246-200110000-00019.
Domenicotti, C., D. Paola, A. Vitali, M. Nitti, C. D’Abramo, D. Cottalasso, G. Maloberti, F. Biasi, G. Poli, E. Chiarpotto, U. M. Marinari, and M. A. Pronzato. 2000. Glutathione depletion induces apoptosis of rat hepatocytes through activationof protein kinase C novel isoforms and dependent increase in AP-1 nuclear binding. Free Radic. Biol. Med. 29:1280–1290 doi:10.1016/S0891-5849(00)00429-9.
Acknowledgments
We thank Prof. Genbao Feng and Mr. Bo Wu for their excellent technical assistance. The study is supported by a grant from special project of Chinese Military Medicine Science and Technology Research”11.5” plan (No. 06Z017).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, P., Li, Y. & Li, J. Protective Roles of Hydroxyethyl Starch 130/0.4 in Intestinal Inflammatory Response and Oxidative Stress After Hemorrhagic Shock and Resuscitation in Rats. Inflammation 32, 71–82 (2009). https://doi.org/10.1007/s10753-009-9105-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-009-9105-7