Abstract
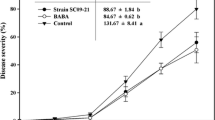
Phytophthora capsici infection of chili pepper seedlings can cause substantial losses due to damping-off and collar rot diseases. Chemical control is no longer effective due to reported resistance development, on top of the related environmental concerns and the consumer demands for reduced use of fungicides. Biological control is a sustainable option, with several agents having been reported to be effective against this pathogen. This research focused on optimizing the application of strain THSW13 of Trichoderma hamatum and a bacterial isolate BJ10–86 with the objectives of improving chili pepper seed germination, reduce damping-off disease incidence, and improve the growth of the seedlings. Bacterial isolate BJ10–86 was subjected to molecular identification and found to be Pseudomonas aeruginosa. Chili pepper seeds treated with the biocontrol agents, individually or in combination, were seeded into commercial nursery media that had been pre-inoculated with P. capsici zoospores. Over a period of 35 days the chili pepper seed treatments significantly (P = 0.008) reduced the disease incidence of seedlings damping-off. Combined application of T. hamatum and P. aeruginosa was the best biocontrol treatment with an area under disease curve of only 36.61 units compared to 92.87 units for the control treatment. Similar results were observed in vitro where T. hamatum and P. aeruginosa synergistically inhibited P. capsici growth by 73.2 %. The inhibition activity of this treatment was similar to mefenoxam treatment, which implies that it is an effective and sustainable alternative for chili pepper seed treatment. The biocontrol seed treatment had no effect on seed germination and seedling growth.


Similar content being viewed by others
References
Alfano, G., Ivey, M. L., Cakir, C., Bos, J. I. B., Miller, S. A., Madden, L. V., Kamoun, S., & Hoitink, H. A. J. (2007). Systemic modulation of gene expression in tomato by Trichoderma hamatum 382. Phytopathology, 97(4), 429–437.
Azadeh, B. F., & Meon, S. (2009). Molecular characterization of Pseudomonas aeruginosa UPM P3 from oil palm rhizosphere. American Journal of Applied Sciences, 6(11), 1915. Available at: http://thescipub.com/pdf/10.3844/ajassp.2009. 1915.1919
Babadoost, M. (2004). Phytophthora blight: a serious threat to cucurbit industries. Urbana, 51, 61801. Available at http://www.apsnet.org/publications/apsnetfeatures/Pages/PhytophthoraBlight.aspx
Babadoost, M., & Islam, S. Z. (2003). Fungicide seed treatment effects on seedling damping-off of pumpkin caused by Phytophthora capsici. Plant Disease, 87(1), 63-68.
Bae, H., Sicher, R. C., Kim, M. S., Kim, S. H., Strem, M. D., Melnick, R. L., & Bailey, B. A. (2009). The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. Journal of Experimental Botany, 60(11), 3279–3295.
Bhakthavatchalu, S., Shivakumar, S., & Sullia, S. B. (2013) Characterization of multiple plant growth promotion traits of Pseudomonas aeruginosa FP6, a potential stress tolerant biocontrol agent. Annals of Biological Research, 4(2), 214–223. Available online at: www.scholarsresearchlibrary.com
Buysens, S., Heungens, K., Poppe, J., & Hofte, M. (1996). Involvement of pyochelin and pyoverdin in suppression of Pythium-induced damping-off of tomato by Pseudomonas aeruginosa 7NSK2. Applied and Environmental Microbiology, 62(3), 865-871.
Castric, P. A. (1975). Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Canadian Journal of Microbiology, 21(5), 613–618. Available at http://www.nrcresearchpress.com/doi/abs/10.1139/m75-088
Chet, I., Harman, G. E., & Baker, R. (1981). Trichoderma hamatum: its hyphal interactions with Rhizoctonia solani and Pythium spp. Microbial Ecology, 7(1), 29–38. Available at http://link.springer.com/article/10.1007/BF02010476#page-1
Cline, E. T., Farr, D. F., & Rossman, A. Y. (2008). A synopsis of Phytophthora with accurate scientific names, host range, and geographic distribution. Plant Health Progress, (March), 0318–01. Available at http://www.plantmanagementnetwork.org/pub/php/review/2008/phytophthora/
Ezziyyani, M., Requena, M. E., Egea-Gilabert, C., Requena, A. M., & Candela, M. E. (2009). Biological control of Phytophthora capsici root rot of pepper (Capsicum annuum) using Burkholderia cepacia and Trichoderma harzianum. Journal of Applied Biosciences, 13, 745-754.
Gevens, A. J., Ando, K., Lamour, K. H., Grumet, R., & Hausbeck, M. K. (2006). A detached cucumber fruit method to screen for resistance to Phytophthora capsici and effect of fruit age on susceptibility to infection. Plant Disease, 90(10), 1276–1282. Available at http://apsjournals.apsnet.org/doi/abs/10.1094/PD-90-1276
Guetsky, R., Shtienberg, D., Elad, Y., & Dinoor, A. (2001). Combining biocontrol agents to reduce the variability of biological control. Phytopathology, 91(7), 621–627.
Haas, D., & Défago, G. (2005). Biological control of soil-borne pathogens by fluorescent pseudomonads. Nature Reviews Microbiology, 3(4), 307-319.
Hu, J. H., Hong, C. X., Stromberg, E. L., & Moorman, G. W. (2008). Mefenoxam sensitivity and fitness analysis of Phytophthora nicotianae isolates from nurseries in Virginia, USA. Plant Pathology, 57(4), 728-736.
Keinath, A. P. (2007). Sensitivity of populations of Phytophthora capsici from South Carolina to mefenoxam, dimethomorph, zoxamide, and cymoxanil. Plant Disease, 91(6), 743–748.
Khan, J., Ooka, J. J., Miller, S. A., Madden, L. V., & Hoitink, H. A. J. (2004). Systemic resistance induced by Trichoderma hamatum 382 in cucumber against Phytophthora crown rot and leaf blight. Plant Disease, 88(3), 280–286.
Kishore, G. K., Pande, S., & Podile, A. R. (2005). Biological control of collar rot disease with broad-spectrum antifungal bacteria associated with groundnut. Canadian Journal of Microbiology, 51(2), 123–132.
Kumar, R. S., Ayyadurai, N., Pandiaraja, P., Reddy, A. V., Venkateswarlu, Y., Prakash, O., & Sakthivel, N. (2005). Characterization of antifungal metabolite produced by a new strain Pseudomonas aeruginosa PUPa3 that exhibits broad‐spectrum antifungal activity and biofertilizing traits. Journal of Applied Microbiology, 98(1), 145-154.
Lamont, I. L., Beare, P. A., Ochsner, U., Vasil, A. I., & Vasil, M. L. (2002). Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences, 99(10), 7072–7077.
Lamour, K. H., Stam, R., Jupe, J., & Huitema, E. (2012). The oomycete broad-host-range pathogen, Phytophthora capsici. Molecular Plant Pathology, 13(4), 329–337.
Leonian, L. H. (1922). Stem and fruit blight of peppers caused by Phytophthora capsici sp. nov. Phytopathology, 12(9), 401–408. Abstract available at http://www.cabdirect.org/abstracts/19231100138.html;jsessionid= 67CCA395449860B2411498AFF7F984D2
Mansoor, F., Sultana, V., & Ehteshamul-Haque, S. (2007). Enhancement of biocontrol potential of Pseudomonas aeruginosa and Paecilomyces lilacinus against root rot of mungbean by a medicinal plant Launaea nudicaulis. L. Pakistan Journal of Botany, 39(6), 2113–2119.
Nautiyal, C. S. (1997). Rhizosphere competence of Pseudomonas sp. NBRI9926 and Rhizobium sp. NBRI9513 involved in the suppression of chickpea (Cicer arietinum L.) pathogenic fungi. FEMS Microbiology Ecology, 23(2), 145–158.
Parra, G., & Ristaino, J. B. (2001). Resistance to mefenoxam and metalaxyl among field isolates of Phytophthora capsici causing Phytophthora blight of bell pepper. Plant Disease, 85(10), 1069–1075.
Pessi, G., & Haas, D. (2001). Dual control of hydrogen cyanide biosynthesis by the global activator GacA in Pseudomonas aeruginosa PAO1. FEMS Microbiology Letters, 200(1), 73–78.
Quimby, P. C., King, L. R., & Grey, W. E. (2002). Biological control as a means of enhancing the sustainability of crop/land management systems. Agriculture, Ecosystems & Environment, 88(2), 147–152.
Raaijmakers, J. M., Sluis, L. V. D., Bakker, P. A., Schippers, B., Koster, M., & Weisbeek, P. J. (1995). Utilization of heterologous siderophores and rhizosphere competence of fluorescent Pseudomonas spp. Canadian Journal of Microbiology, 41(2), 126–135.
Ristaino, J. B., & Johnston, S. A. (1999). Ecologically based approaches to management of Phytophthora blight on bell pepper. Plant Disease, 83(12), 1080–1089.
Roberts, D. P., Lohrke, S. M., Meyer, S. L., Buyer, J. S., Bowers, J. H., Jacyn Baker, C., Li, W., de Souza, J. T., Lewis, J. A., & Chung, S. (2005). Biocontrol agents applied individually and in combination for suppression of soilborne diseases of cucumber. Crop Protection, 24(2), 141–155.
Saikia, R., Kumar, R., Arora, D. K., Gogoi, D. K., & Azad, P. (2006). Pseudomonas aeruginosa inducing rice resistance against Rhizoctonia solani: production of salicylic acid and peroxidases. Folia Microbiologica, 51(5), 375–380.
Schisler, D. A., Slininger, P. J., & Bothast, R. J. (1997). Effects of antagonist cell concentration and two-strain mixtures on biological control of Fusarium dry rot of potatoes. Phytopathology, 87(2), 177–183.
Shizrad, A., Fallahzadeh-Mamaghani, V., & Pazhouhandeh, M. (2012). Antagonistic potential of fluorescent Pseudomonads and control of crown and root rot of cucumber caused by Phytophthora drechslera. Plant Pathology Journal,28(1), 1–9. Available online at: http://10.5423/PPJ.OA.05.2011.0100
Shukla, Y. R., Chhopal, T., & Sharma, R. (2011). Effect of age of transplants on growth and yield of capsicum. International Journal of Farm Sciences, 1(2), 56–62. Available at http://210.212.129.138/ojs/index.php/IJFS/article/view/805
Spadaro, D., & Gullino, M. L. (2005). Improving the efficacy of biocontrol agents against soilborne pathogens. Crop Protection, 24(7), 601–613.
Steyaert, J. M., Stewart, A., Jaspers, M. V., Carpenter, M., & Ridgway, H. J. (2004). Co-expression of two genes, a chitinase (chit42) and proteinase (prb1), implicated in mycoparasitism by Trichoderma hamatum. Mycologia, 96(6), 1245–1252.
Studholme, D. J., Harris, B., Le Cocq, K., Winsbury, R., Perera, V., Ryder, L., Ward, J. L., Beale, M. H., Thornton, C. R.,& Grant, M. (2013). Investigating the beneficial traits of Trichoderma hamatum GD12 for sustainable agriculture - insights from genomics. Frontiers in Plant Science, 4(258), 1–13. Available online at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3726867/
Sulochana, M. B., Jayachandra, S. Y., Kumar, S. K., & Dayanand, A. (2014). Antifungal attributes of siderophore produced by the Pseudomonas aeruginosa JAS-25.Journal of. Basic Microbiology, 54(5), 418–424. doi:10.1002/jobm.201200770.
Thilagavathi, R., Saravanakumar, D., Ragupathi, N., & Samiyappan, R. (2007). A combination of biocontrol agents improves the management of dry root rot (Macrophomina phaseolina) in greengram. Phytopathologia Mediterranea, 46(2), 157–167.
Tian, D., & Babadoost, M. (2004). Host range of Phytophthora capsici from pumpkin and pathogenicity of isolates. Plant Disease, 88(5), 485–489.
Xu, X. M., Jeffries, P., Pautasso, M., & Jeger, M. J. (2011). A numerical study of combined use of two biocontrol agents with different biocontrol mechanisms in controlling foliar pathogens. Phytopathology, 101(9), 1032–1044.
Yobo, K. S., Laing, M. D., & Hunter, C. H. (2010). Application of selected biological control agents in conjunction with tolclofos-methyl for the control of damping-off caused by Rhizoctonia solani. African Journal of Biotechnology, 9(12), 1789–1796. Available at: http://www.ajol.info/index.php/ajb/-article/view/78366/68729
Zhou, X., Ye, L., & Wu, J. C. (2013). Efficient production of l-lactic acid by newly isolated thermophilic Bacillus coagulans WCP10-4 with high glucose tolerance. Applied Microbiology and Biotechnology, 97(10), 4309–4314.
Acknowledgments
The authors would like to acknowledge and the assistance of laboratory and field staff of the Plant Protection Department, Bogor Agricultural University, Indonesia. Special gratitude goes to Dr. Suryo Wiyono for donating the T. hamatum isolate THSW13 used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chemeltorit, P.P., Mutaqin, K.H. & Widodo, W. Combining Trichoderma hamatum THSW13 and Pseudomonas aeruginosa BJ10–86: a synergistic chili pepper seed treatment for Phytophthora capsici infested soil. Eur J Plant Pathol 147, 157–166 (2017). https://doi.org/10.1007/s10658-016-0988-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-0988-5