Summary
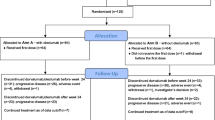
The novel oral antiangiogenic agent TSU-68 was investigated in patients with metastatic breast cancer. Patients with anthracycline-pretreated metastatic breast cancer were randomly assigned to receive either TSU-68 400 mg twice daily on days 1–21 plus docetaxel 60 mg/m2 on day 1 every 3 weeks, or docetaxel 60 mg/m2 on day 1 every 3 weeks. The primary endpoint was progression-free survival. Between November 2006 and December 2007, 81 patients were included in this study (41 for TSU-68 plus docetaxel and 40 for docetaxel alone). Median progression-free survival was 6.8 months (95 % confidence interval [CI] = 5.4–12.5 months) in the TSU-68 plus docetaxel group and 8.1 months (95 % CI = 4.0–13.7 months) in the docetaxel-alone group (hazard ratio [HR] = 1.0; 95 % CI = 0.6–1.8; p = 0.95). There were no significant differences in the overall response rates and overall survival between groups (p = 0.29 and p = 0.42, respectively). In subgroup analysis, TSU-68 plus docetaxel was associated with better overall survival than docetaxel alone in anthracycline-resistant patients (HR = 0.3; 95 % CI = 0.1–0.8; p = 0.02). The most frequent adverse events were neutropenia and anorexia in both arms. Although both regimens were well tolerated, grade 3/4 non-hematologic toxicity was more frequently observed in the TSU-68 plus docetaxel group. Combination of TSU-68 and docetaxel is well tolerated but failed to demonstrate superior efficacy over docetaxel alone in anthracycline-pretreated breast cancer patients. As TSU-68 was associated with better survival in the anthracycline-resistant subgroup, it should be further explored in this subgroup.



Similar content being viewed by others
References
Chia SK, Speers CH, D’yachkova Y, Kang A, Malfair-Taylor S, Barnett J, Coldman A, Gelmon KA, O’reilly SE, Olivotto IA (2007) The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer 110(5):973–979. doi:10.1002/cncr.22867
Harvey V, Mouridsen H, Semiglazov V, Jakobsen E, Voznyi E, Robinson BA, Groult V, Murawsky M, Cold S (2006) Phase III trial comparing three doses of docetaxel for second-line treatment of advanced breast cancer. J Clin Oncol 24(31):4963–4970
Hicklin DJ, Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23(5):1011–1027
Gasparini G, Longo R, Fanelli M, Teicher BA (2005) Combination of antiangiogenic therapy with other anticancer therapies: results, challenges, and open questions. J Clin Oncol 23(6):1295–1311
Dvorak HF, Brown LF, Detmar M, Dvorak AM (1995) Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146(5):1029–1039
Reddy S, Raffin M, Kaklamani V (2012) Targeting angiogenesis in metastatic breast cancer. Oncologist 17(8):1014–1026
Laird AD, Vajkoczy P, Shawver LK, Thurnher A, Liang C, Mohammadi M, Schlessinger J, Ullrich A, Hubbard SR, Blake RA, Fong TA, Strawn LM, Sun L, Tang C, Hawtin R, Tang F, Shenoy N, Hirth KP, McMahon G, Cherrington (2000) SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Res 60(15):4152–4160
Machida S, Saga Y, Takei Y, Mizuno I, Takayama T, Kohno T, Konno R, Ohwada M, Suzuki M (2005) Inhibition of peritoneal dissemination of ovarian cancer by tyrosine kinase receptor inhibitor SU6668 (TSU-68). Int J Cancer 114(2):224–229. doi:10.1002/ijc.20751
Naumova E, Ubezio P, Garofalo A, Borsotti P, Cassis L, Riccardi E, Scanziani E, Eccles SA, Bani MR, Giavazzi R (2006) The vascular targeting property of paclitaxel is enhanced by SU6668, a receptor tyrosine kinase inhibitor, causing apoptosis of endothelial cells and inhibition of angiogenesis. Clin Cancer Res 12(6):1839–1849
Yonekura K, Basaki Y, Fujita H, Chikahisa L, Hashimoto A, Cherrington J, Shavwer L, Yamada Y, Kitazata K (2001) Inhibition of VEGF/KDR signaling by TSU-68 (SU6668), an oral anti-angiogenic agent, can synergistically enhance the anti-tumor activity of taxol; a new paradigm for breast cancer chemotherapy. Breast Cancer Res Treat 69:216, abstr 29
Murakami H, Ueda Y, Shimoyama T, Yamamoto N, Yamada Y, Arioka H, Tamura T (2011) Phase I, pharmacokinetic, and biological studies of TSU-68, a novel multiple receptor tyrosine kinase inhibitor, administered after meals with solid tumors. Cancer Chemother Pharmacol 67(5):1119–1128. doi:10.1007/s00280-010-1405-y
Suzuki Y, Saeki T, Aogi K, Toi M, Fujii H, Inoue K, Watanabe T, Fujiwara Y, Ito Y, Takatsuka Y, Iwata H, Arioka H, Tokuda Y (2013) A multicenter phase II study of TSU-68, a novel oral multiple tyrosine kinase inhibitor, in patients with metastatic breast cancer progressing despite prior treatment with an anthracycline-containing regimen and taxane. Int J Clin Oncol 18(4):590–597. doi:10.1007/s10147-012-0421-9
Toi M, Saeki T, Iwata H, Inoue K, Tokuda Y, Sato Y, Ito Y, Aogi K, Takatsuka Y, Arioka H (2012) A multicenter phase II study of TSU-68, an oral multiple tyrosine kinase inhibitor, in combination with docetaxel in metastatic breast cancer patients with anthracycline resistance. Breast Cancer. doi:10.1007/s12282-012-0344-3
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Barrios CH, Liu MC, Lee SC, Vanlemmens L, Ferrero JM, Tabei T, Pivot X, Iwata H, Aogi K, Lugo-Quintana R, Harbeck N, Brickman MJ, Zhang K, Kern KA, Martin M (2010) Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Res Treat 121(1):121–131. doi:10.1007/s10549-010-0788-0
Bergh J, Bondarenko IM, Lichinitser MR, Liljegren A, Greil R, Voytko NL, Makhson AN, Cortes J, Lortholary A, Bischoff J, Chan A, Delaloge S, Huang X, Kern KA, Giorgetti C (2012) First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: results of a prospective, randomized phase III study. J Clin Oncol 30(9):921–929
Robert NJ, Saleh MN, Paul D, Generali D, Gressot L, Copur MS, Brufsky AM, Minton SE, Giguere JK, Smith JW, Richards PD, Gernhardt D, Huang X, Liau KF, Kern KA, Davis J (2011) Sunitinib plus paclitaxel versus bevacizumab plus paclitaxel for first-line treatment of patients with advanced breast cancer: a phase III, randomized, open-label trial. Clin Breast Cancer 11(2):82–92
Crown J, Dieras V, Staroslawska E, Yardley D, Davidson N, Bachelot T, Tassell V, Huang X, Kern K, Romieu G (2010) Phase III trial of sunitinib (SU) in combination with capecitabine (C) versus C in previously treated advanced breast cancer (ABC). J Clin Oncol 28(18 suppl), abstr LBA1011
Baselga J, Segalla JG, Roche H, Del Giglio A, Pinczowski H, Ciruelos EM, Filho SC, Gomez P, Van Eyll B, Bermejo B, Llombart A, Garicochea B, Duran MA, Hoff PM, Espie M, de Moraes AA, Ribeiro RA, Mathias C, Gil Gil M, Ojeda B, Morales J, Kwon Ro S, Li S, Costa F (2012) Sorafenib in combination with capecitabine: an oral regimen for patients with HER2-negative locally advanced or metastatic breast cancer. J Clin Oncol 30(13):1484–1491
Hudis C, Tauer K, Hermann R, Makari-Judson G, Isaacs C, Beck J (2011) Sorafenib (SOR) plus chemotherapy (CRx) for patients (pts) with advanced (adv) breast cancer (BC) previously treated with bevacizumab (BEV). J Clin Oncol 29:1009
Ueda Y, Shimoyama T, Murakami H, Yamamoto N, Yamada Y, Arioka H, Tamura T (2011) Phase I and pharmacokinetic study of TSU-68, a novel multiple receptor tyrosine kinase inhibitor, by twice daily oral administration between meals in patients with advanced solid tumors. Cancer Chemother Pharmacol 67(5):1101–1109. doi:10.1007/s00280-010-1404-z
Kanai F, Yoshida H, Tateishi R, Sato S, Kawabe T, Obi S, Kondo Y, Taniguchi M, Tagawa K, Ikeda M, Morizane C, Okusaka T, Arioka H, Shiina S, Omata M (2011) A phase I/II trial of the oral antiangiogenic agent TSU-68 in patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol 67(2):315–324. doi:10.1007/s00280-010-1320-2
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357(26):2666–2676
Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, Phan SC, O’Shaughnessy J (2011) RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 29(10):1252–1260
Bondarde S, Kaklamani V, Sahoo TP, Lokanatha D, Raina V, Jain M, Schwartzberg L, Gradishar WJ (2010) Sorafenib in combination with paclitaxel as a first-line therapy in patients with locally recurrent or metastatic breast cancer: overall survival results from a double-blind, randomized, placebo-controlled, phase 2b trial. Cancer Res 70(24 suppl), abstr P2-16-03
Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, Harbeck N, Steger GG, Schneeweiss A, Wardley AM, Chlistalla A, Romieu G (2010) Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 28(20):3239–3247
Mariani G, Burdaeva O, Roman L, Staroslawska E, Udovitsa D, Driol P, Goisis G, Zamagni C, Semiglazov V, Gianni L (2011) A double-blind, randomized phase lib study evaluating the efficacy and safety of sorafenib (SOR) compared to placebo (PL) when administered in combination with docetaxel and/or letrozole in patients with metastatic breast cancer (MBC): FM-B07-01 Trial. Eur J Cancer 47:10
Rugo HS, Stopeck AT, Joy AA, Chan S, Verma S, Lluch A, Liau KF, Kim S, Bycott P, Rosbrook B, Bair AH, Soulieres D (2011) Randomized, placebo-controlled, double-blind, phase II study of axitinib plus docetaxel versus docetaxel plus placebo in patients with metastatic breast cancer. J Clin Oncol 29(18):2459–2465
Disclosure
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
S.-B. Kim and C. Yoo contributed equally to this work and should be considered as first co-authors.
Rights and permissions
About this article
Cite this article
Kim, SB., Yoo, C., Ro, J. et al. Combination of docetaxel and TSU-68, an oral antiangiogenic agent, in patients with metastatic breast cancer previously treated with anthracycline: Randomized phase II multicenter trial. Invest New Drugs 32, 753–761 (2014). https://doi.org/10.1007/s10637-014-0093-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0093-6