Abstract
Background
Early detection and removal of precursor lesions reduce colorectal cancer morbidity and mortality. Sessile serrated adenomas/polyps (SSP) are a recognized precursor of cancer, but there are limited studies on whether current screening techniques detect this pathology.
Aims
To investigate the sensitivity of fecal immunochemical tests (FIT) and epigenetic biomarkers in blood for detection of SSP.
Methods
A prospective study offered FIT and a blood test (Colvera for methylated BCAT1 and IKZF1) to adults referred for colonoscopy. Sensitivity of FIT and the blood test were determined for four types of pathology: low-risk conventional adenoma, high-risk adenoma, SSP, and absence of neoplasia. Comparisons were made for FIT positivity at 10 and 20 μg hemoglobin (Hb)/g feces.
Results
One thousand eight hundred and eighty-two subjects completed FIT and underwent colonoscopy. One thousand four hundred and three were also tested for methylated BCAT1/IKZF1. The sensitivity of FIT (20 μg Hb/g feces) for SSP was 16.3%. This was lower than the sensitivity for high-risk adenomas (28.7%, p < 0.05), but no different to that for low-risk adenomas (13.1%) or no neoplasia (8.4%). A positive FIT result for SSP was not associated with demographics, morphology, concurrent pathology or intake of medications that increase bleeding risk. FIT sensitivity for SSP did not significantly increase through lowering the positivity threshold to 10 μg Hb/g feces (20.4%, p > 0.05). Sensitivity of the blood test for SSP was 8.8%, and 26.5% when combined with FIT.
Conclusions
Both FIT and blood-based markers of DNA hypermethylation have low sensitivity for detection of SSP. Further development of sensitive screening tests is warranted.



Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. https://doi.org/10.1002/ijc.29210.
Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251–2270.
Eide TJ. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int J Cancer. 1986;38:173–176.
Lew J-B, St John DJB, Xu X-M, et al. Long-term evaluation of benefits, harms, and cost-effectiveness of the National Bowel Cancer Screening Program in Australia: a modelling study. Lancet Pub Health. 2017;2:e331–340.
Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–1649. https://doi.org/10.1136/gutjnl-2014-309086.
Cole SR, Tucker GR, Osborne JM, et al. Shift to earlier stage at diagnosis as a consequence of the National Bowel Cancer Screening Program. Med J Aust. 2013;198:327–330.
Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. https://doi.org/10.1056/nejm199312303292701.
Chiu HM, Chen SL, Yen AM, et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the One Million Taiwanese Screening Program. Cancer. 2015;121:3221–3229. https://doi.org/10.1002/cncr.29462.
Zorzi M, Fedeli U, Schievano E, et al. Impact on colorectal cancer mortality of screening programmes based on the faecal immunochemical test. Gut. 2015;64:784–790. https://doi.org/10.1136/gutjnl-2014-307508.
Fenocchi E, Gaggero P, Rondán M, et al. Usefulness of the fecal immunochemical test in the detection of advanced adenomas in subjects at average risk for colorectal cancer. Endoscopia. 2015;27:64–68.
Digby J, Fraser CG, Carey FA, et al. Faecal haemoglobin concentration is related to severity of colorectal neoplasia. J Clin Pathol. 2013;66:415–419. https://doi.org/10.1136/jclinpath-2013-201445.
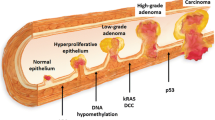
Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138:2088–2100. https://doi.org/10.1053/j.gastro.2009.12.066.
Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. https://doi.org/10.1111/j.1365-2559.2006.02549.x.
Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA. 1999;96:8681–8686.
Bettington M, Walker N, Rosty C, et al. Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma. Gut. 2017;66:97–106. https://doi.org/10.1136/gutjnl-2015-310456.
Singh S, Singh PP, Murad MH, Singh H, Samadder NJ. Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1375–1389. https://doi.org/10.1038/ajg.2014.171.
Hassan C, Quintero E, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842–851. https://doi.org/10.1055/s-0033-1344548.
Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. https://doi.org/10.1053/j.gastro.2012.06.001.
Chang LC, Shun CT, Hsu WF, et al. Fecal immunochemical test detects sessile serrated adenomas and polyps with a low level of sensitivity. Clin Gastroenterol Hepatol. 2017;15:872–879. https://doi.org/10.1016/j.cgh.2016.07.029.
Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287–1297. https://doi.org/10.1056/nejmoa1311194.
Symonds EL, Osborne JM, Cole SR, Bampton PA, Fraser RJ, Young GP. Factors affecting faecal immunochemical test positive rates: demographic, pathological, behavioural and environmental variables. J Med Screen. 2015;. https://doi.org/10.1177/0969141315584783.
Kahi CJ. How does the serrated polyp pathway alter CRC screening and surveillance? Dig Dis Sci. 2015;60:773–780. https://doi.org/10.1007/s10620-014-3449-z.
Park SJ, Rashid A, Lee JH, Kim SG, Hamilton SR, Wu TT. Frequent CpG island methylation in serrated adenomas of the colorectum. Am J Pathol. 2003;162:815–822. https://doi.org/10.1016/s0002-9440(10)63878-3.
Symonds EL, Pedersen SK, Murray DH, et al. Circulating tumour DNA for monitoring colorectal cancer-a prospective cohort study to assess relationship to tissue methylation, cancer characteristics and surgical resection. Clin Epigenetics. 2018;10:63. https://doi.org/10.1186/s13148-018-0500-5.
Symonds EL, Pedersen SK, Baker RT, et al. A blood test for methylated BCAT1 and IKZF1 vs. a fecal immunochemical test for detection of colorectal neoplasia. Clin Transl Gastroenterol. 2016;7:e137. https://doi.org/10.1038/ctg.2015.67.
Bosman F, World Health Organization. Who Classification of Tumours of the Digestive System. 4th ed. Lyon: International Agency for Research on Cancer; 2010.
Pedersen SK, Symonds EL, Baker RT, et al. Evaluation of an assay for methylated BCAT1 and IKZF1 in plasma for detection of colorectal neoplasia. BMC Cancer. 2015;15:654. https://doi.org/10.1186/s12885-015-1674-2.
Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160:171. https://doi.org/10.7326/m13-1484.
Lane JM, Chow E, Young GP, et al. Interval fecal immunochemical testing in a colonoscopic surveillance program speeds detection of colorectal neoplasia. Gastroenterology. 2010;139:1918–1926. https://doi.org/10.1053/j.gastro.2010.08.005.
Pedersen SK, Baker RT, McEvoy A, et al. A two-gene blood test for methylated DNA sensitive for colorectal cancer. PLoS ONE. 2015;10:e0125041.
IJspeert J, Bevan R, Senore C, et al. Detection rate of serrated polyps and serrated polyposis syndrome in colorectal cancer screening cohorts: a European overview. Gut. 2017;66:1225–1232. https://doi.org/10.1136/gutjnl-2015-310784.
Suehiro Y, Hashimoto S, Higaki S, et al. Blood free-circulating DNA testing by highly sensitive methylation assay to diagnose colorectal neoplasias. Oncotarget. 2018;9:16974–16987. https://doi.org/10.18632/oncotarget.24768.
Ma MX, Bourke MJ. Sessile serrated adenomas: how to detect, characterize and resect. Gut Liver. 2017;11:747–760. https://doi.org/10.5009/gnl16523.
Kambara T, Simms LA, Whitehall VL, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53:1137–1144. https://doi.org/10.1136/gut.2003.037671.
Symonds E, Anwar S, Young G, et al. Sessile serrated polyps with synchronous conventional adenomas increase risk of future advanced neoplasia. Dig Dis Sci. (Epub ahead of print). https://doi.org/10.1007/s10620-019-5454-8.
van Roon AH, Wilschut JA, Hol L, et al. Diagnostic yield improves with collection of 2 samples in fecal immunochemical test screening without affecting attendance. Clin Gastroenterol Hepatol. 2011;9:333–339. https://doi.org/10.1016/j.cgh.2010.12.012.
Acknowledgment
R Meng was supported by a Grant funded by the financial support of Cancer Council SA’s Beat Cancer Project on behalf of its donors and the State Government of South Australia through the Department of Health together with the support of the Flinders Medical Centre Foundation, its donors and partners.
Funding
This study was funded in part by the National Health and Medical Research Council (APP1006242 and APP1017083) and Clinical Genomics Pty Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Scientists from Clinical Genomics Pty Ltd conducted the blood assays (blinded to all clinical outcomes) and assisted with the writing of the manuscript. Fecal immunochemical tests were provided by Eiken Chemical Company, Tokyo, Japan, but they had no influence on study design, analysis or the decision to submit the manuscript for publication. Author G Young is a paid consultant of Clinical Genomics Pty Ltd. S Pedersen is employed by Clinical Genomics Pty Ltd.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cock, C., Anwar, S., Byrne, S.E. et al. Low Sensitivity of Fecal Immunochemical Tests and Blood-Based Markers of DNA Hypermethylation for Detection of Sessile Serrated Adenomas/Polyps. Dig Dis Sci 64, 2555–2562 (2019). https://doi.org/10.1007/s10620-019-05569-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05569-8