Abstract
Background
Sporadic nonampullary duodenal epithelial tumors (NADETs) are uncommon, and thus their clinicopathological features have not been fully assessed.
Aims
In this study, we have analyzed a series of early sporadic NADETs, focusing on various immunohistological features.
Methods
We conducted a multicenter retrospective analysis of 68 patients with endoscopically resected sporadic NADETs. Associations between immunohistological features and clinicopathological features were statistically analyzed.
Results
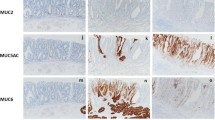
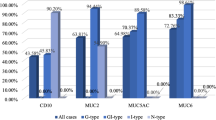
The 68 patients consisted of 46 men (68%) and 22 women (32%) with a mean age of 60.7 ± 12.2 years (range 37–85 years). The 68 tumors were composed of 39 adenomas (57%) and 29 early-stage adenocarcinomas (43%). Duodenal adenocarcinomas were larger in size than adenomas and had papillary architecture in their pathological diagnosis with statistical significance. Duodenal adenocarcinomas also demonstrated a significantly higher expression of gastric markers (MUC5AC and MUC6) and a higher MIB-1 index. Duodenal adenomas were contrastively apt to express intestinal markers (MUC2, CDX1 and CDX2). Of the 68 cases analyzed, there were only 3 tumors positive for p53 staining, all of which were adenocarcinoma. When 7 submucosal invasive cancers and 21 intramucosal cancers were compared, submucosal invasion was positively associated with expression of MUC5AC. Also, submucosal invasion showed strong association with double-positivity of MUC5AC and MUC6.
Conclusions
Our results indicate that immunohistochemical evaluation is useful for predicting malignant potential of NADETs, especially focusing on the expression of gastrointestinal markers.


Similar content being viewed by others
References
Neugut AI, Jacobson JS, Suh S, et al. The epidemiology of cancer of the small bowel. Cancer Epidemiol Biomark Prev. 1998;7:243–251.
Poultsides GA, Huang LC, Cameron JL, et al. Duodenal adenocarcinoma: clinicopathologic analysis and implications for treatment. Ann Surg Oncol. 2012;19:1928–1935.
Aparicio T, Zaanan A, Svrcek M, et al. Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis. 2014;46:97–104.
Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63–71.
Lu Y, Fröbom R, Lagergren J. Incidence patterns of small bowel cancer in a population-based study in Sweden: increase in duodenal adenocarcinoma. Cancer Epidemiol. 2012;36:e158–e163.
Bosman FT, Carneiro F, Hruban RH, Theise ND, eds. WHO Classification of Tumours of the Digestive System, 4th edn.
Fernández-Esparrach G, Calderón A, de la Peña J, et al. Endoscopic submucosal dissection. Endoscopy. 2014;46:361–370.
Obata S, Suenaga M, Araki K, et al. Use of strip biopsy in a case of early duodenal cancer. Endoscopy. 1992;24:232–234.
Abbass R, Rigaux J, Al-Kawas FH. Nonampullary duodenal polyps: characteristics and endoscopic management. Gastrointest Endosc. 2010;71:754–759.
Maruoka D, Arai M, Kishimoto T, et al. Clinical outcomes of endoscopic resection for nonampullary duodenal high-grade dysplasia and intramucosal carcinoma. Endoscopy. 2013;45:138–141.
Sohn JW, Jeon SW, Cho CM, et al. Endoscopic resection of duodenal neoplasms: a single-center study. Surg Endosc. 2010;24:3195–3200.
Nonaka S, Oda I, Tada K, et al. Clinical outcome of endoscopic resection for nonampullary duodenal tumors. Endoscopy. 2015;47:129–135.
Takimoto K, Imai Y, Matsuyama K. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to prevent delayed perforation after duodenal endoscopic submucosal dissection. Dig Endosc. 2014;26:46–49.
Matsumoto S, Miyatani H, Yoshida Y. Future directions of duodenal endoscopic submucosal dissection. World J Gastrointest Endosc. 2015;7:389–395.
Bourke MJ. Endoscopic resection in the duodenum: current limitations and future directions. Endoscopy. 2013;45:127–132.
Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1–11.
Kakushima N, Kanemoto H, Sasaki K, et al. Endoscopic and biopsy diagnoses of superficial, nonampullary, duodenal adenocarcinomas. World J Gastroenterol. 2015;21:5560–5567.
Goda K, Kikuchi D, Yamamoto Y, et al. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan: multicenter case series. Dig Endosc. 2014;26:23–29.
Japanese GCA. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113–123.
Okada K, Fujisaki J, Kasuga A, et al. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: a study of follow-up surveillance. Am J Gastroenterol. 2010;106:357–364.
Maruoka D, Arai M, Ishigami H, et al. Sporadic nonampullary duodenal adenoma/carcinoma is associated with not only colon adenoma/carcinoma but also gastric cancer: association of location of duodenal lesions with comorbid diseases. Scand J Gastroenterol. 2015;50:333–340.
Yamamichi N, Inada K, Ichinose M, et al. Frequent loss of Brm expression in gastric cancer correlates with histologic features and differentiation state. Cancer Res. 2007;67:10727–10735.
Konno-Shimizu M, Yamamichi N, Inada K, et al. Cathepsin E is a marker of gastric differentiation and signet-ring cell carcinoma of stomach: a novel suggestion on gastric tumorigenesis. PLoS ONE. 2013;8:e56766.
Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–1664.
Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212–7220.
Zhang S, Cui Y, Zhong B, et al. Clinicopathological characteristics and survival analysis of primary duodenal cancers: a 14-year experience in a tertiary centre in South China. Int J Colorectal Dis. 2011;26:219–226.
Oka S, Tanaka S, Nagata S, et al. Clinicopathologic features and endoscopic resection of early primary nonampullary duodenal carcinoma. J Clin Gastroenterol. 2003;37:381–386.
Ushiku T, Arnason T, Fukayama M, et al. Extra-ampullary duodenal adenocarcinoma. Am J Surg Pathol. 2014;38:1484–1493.
Solaini L, Jamieson NB, Metcalfe M, et al. Outcome after surgical resection for duodenal adenocarcinoma in the UK. Br J Surg. 2015;102:676–681.
Park SM, Ham JH, Kim BW, et al. Feasibility of endoscopic resection for sessile nonampullary duodenal tumors: a multicenter retrospective study. Gastroenterol Res Pract. 2015;2015:692492.
Arai T, Murata T, Sawabe M, et al. Primary adenocarcinoma of the duodenum in the elderly: clinicopathological and immunohistochemical study of 17 cases. Pathol Int. 1999;49:23–29.
Bartman AE, Buisine MP, Aubert JP, et al. The MUC6 secretory mucin gene is expressed in a wide variety of epithelial tissues. J Pathol. 1998;186:398–405.
Ushiku T, Arnason T, Ban S, et al. Very well-differentiated gastric carcinoma of intestinal type: analysis of diagnostic criteria. Mod Pathol. 2013;26:1620–1631.
Saleh HA, Aburashed A, Bober P, et al. P53 protein immunohistochemical expression in colonic adenomas with and without associated carcinoma. Am J Gastroenterol. 1998;93:980–984.
Hong MK, Laskin WB, Herman BE, et al. Expansion of the Ki-67 proliferative compartment correlates with degree of dysplasia in Barrett’s esophagus. Cancer. 1995;75:423–429.
Matsumoto T, Iida M, Nakamura S, et al. Depressed adenoma of the duodenum in patients with familial adenomatous polyposis: endoscopic and immunohistochemical features. Cancer. 1999;86:1414–1420.
Kushima R, Stolte M, Dirks K, et al. Gastric-type adenocarcinoma of the duodenal second portion histogenetically associated with hyperplasia and gastric-foveolar metaplasia of Brunner’s glands. Virchows Arch. 2002;440:655–659.
Kushima R, Ruthlein HJ, Stolte M, et al. ‘Pyloric gland-type adenoma’ arising in heterotopic gastric mucosa of the duodenum, with dysplastic progression of the gastric type. Virchows Arch. 1999;435:452–457.
Yamamichi N, Inada K, Furukawa C, et al. Cdx2 and the Brm-type SWI/SNF complex cooperatively regulate villin expression in gastrointestinal cells. Exp Cell Res. 2009;315:1779–1789.
Zhang HY, Spechler SJ, Souza RF. Esophageal adenocarcinoma arising in Barrett esophagus. Cancer Lett. 2009;275:170–177.
Acknowledgments
The authors thank Dr. Yosuke Muraki from Wakayama Medical University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Minatsuki, C., Yamamichi, N., Inada, Ki. et al. Expression of Gastric Markers Is Associated with Malignant Potential of Nonampullary Duodenal Adenocarcinoma. Dig Dis Sci 63, 2617–2625 (2018). https://doi.org/10.1007/s10620-018-5179-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5179-0