Abstract
Background
Recent evidence indicates that transplanted autologous bone marrow cells (BMCs) can be converted into functional liver cells. BMC therapy can improve hepatic function and increase the potential for liver regeneration in patients with serious liver damage. We investigated whether BMC therapy influenced liver regeneration after massive hepatectomy in mice.
Methods
Male C57/BL6 mice underwent 70 % hepatectomy, followed by injection of BMCs via the portal vein (PV group), BMCs via the tail vein (IV group), or saline via the portal vein (control group). Analysis of serum enzyme levels and liver histology was performed on postoperative days (POD) 1, 3, and 5.
Results
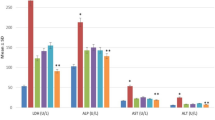
Compared with the control group, the rate of liver regeneration on POD 3 and 5 was significantly higher in the PV group, but not in the IV group. Examination of the mitotic index and Ki-67 labeling index revealed that the increased liver regeneration resulted from stimulation of DNA synthesis. On POD 3, the serum levels of interleukin (IL)-6 and hepatocyte growth factor (HGF) were significantly higher and the expression of IL-6 and HGF mRNA in the remnant liver tended to be higher in the PV group than in the control group. Histological examination showed BMCs in the liver of the PV group, as well as conversion of BMCs into liver cells.
Conclusions
Our findings indicate that the injection of BMCs via the portal vein, but not the injection of BMCs via the tail, enhances liver regeneration after massive hepatectomy in mice.






Similar content being viewed by others
References
Stutchfield BM, Rashid S, Forbes SJ, et al. Practical barriers to delivering autologous bone marrow stem cell therapy as an adjunct to liver resection. Stem Cell Dev. 2010;19:155–161.
Yousef M, Shannwell CM, Kostering M, et al. The BALANCE study: clinical benefit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infraction. J Am Coll Cardiol. 2009;53:2262–2269.
Flohr TR, Bonatti H Jr, Brayman KL, et al. The use of stem cells in liver disease. Curr Opion Organ Transplant. 2009;14:64–71.
Newsome PN, Houlihan DD. Critical review of clinical trials of bone marrow stem cells in liver disease. Gastroenterology. 2008;135:438–450.
Kaibori M, Adachi Y, Shimo T, et al. Stimulation of liver regeneration after hepatectomy in mice by injection of bone marrow mesenchymal stem cells via the portal vein. Transplant Proc. 2012;44:1107–1109.
Mohamadnejad M, Namiri M, Bagheri M, et al. Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol. 2007;13:3359–3363.
Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10:459–466.
Higgins GM, Anderson RM. Experimental pathology of the liver: restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202.
Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159.
Kaibori M, Kawa SKH, Ishizaki M, et al. HA/GSA-Rmax ratio as a predictor of postoperative liver failure. World J Surg. 2008;32:2410–2418.
Am Esch JS II, Knoefel WT, Klein M, et al. Portal application of autologous CD133+ bone marrow cells to the liver: a novel concept to support hepatic regeneration. Stem Cells. 2005;23:463–470.
Terai S, Sakaida I. Current status of autologous bone marrow cell infusion therapy for liver cirrhosis patients. Hepatol Res. 2008;38:S72–S75.
Fan TX, Hisha H, Jin TN, et al. Successful allogeneic bone marrow transplantation (BMT) by injection of bone marrow cells via portal vein: stromal cells as BMT-facilitating cells. Stem Cells. 2001;19:144–150.
Wang X, Willenbring H, Akkari Y, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901.
Vassilopoulos G, Wang PR, Russell DW, et al. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904.
Jang YY, Collector MI, Baylin SB, et al. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532–539.
Petersen BE, Bowen WC, Patrene KD, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170.
De Vos R, Desmet V. Ultrastructural characteristics of novel epithelial cell types identified in human patologic liver specimens with chronic ductular reaction. Am J Pathol. 1992;140:1441–1450.
Golding M, Sarraf CE, Lalani EN, et al. Oval cell differentiation into hepatocytes in the acetylaminofluorene-treated regenerating rat liver. Hepatology. 1995;22:1243–1253.
Yin L, Lynch D, Sell S. Participation of different cell types in the restitutive response of the rat liver to periportal injury induced by allyl alcohol. J Hepatol. 1999;31:497–507.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaibori, M., Adachi, Y., Shimo, T. et al. Bone Marrow Cells Enhance Liver Regeneration After Massive Hepatectomy in Mice. Dig Dis Sci 59, 1484–1489 (2014). https://doi.org/10.1007/s10620-014-3032-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3032-7